Structure and chemical durability improvement of alkali silicate glass by zirconium dioxide and erbium oxide addition
DOI:
https://doi.org/10.55713/jmmm.v33i4.1624Keywords:
Chemical durability, Charge compensator, X-ray absorption spectroscopy, Rare earth oxide containing glass, Glass structureAbstract
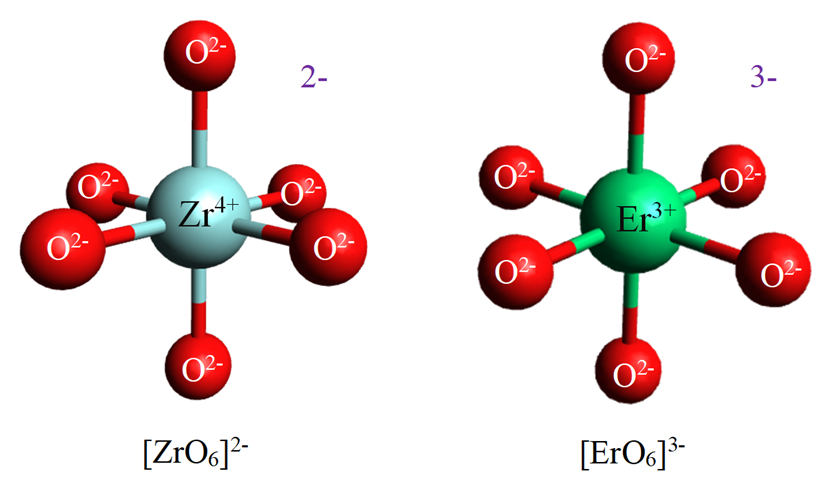
Glass structure tailoring of alkali silicate glasses by addition of ZrO2 and Er2O3 is found to enhance the chemical durability of glasses. ZrO2 (x ranged between 5 mol% to 15 mol%) and Er2O3 (y ranged between 0.5 mol% to 1.5 mol%) were used to replace SiO2 and Na2O, respectively, in the glasses with the nominal composition of 10Li2O-(15-y)Na2O-10CaO-(65-x)SiO2-xZrO2-y Er2O3. The samples were prepared by conventional melt quenching technique. The structures of produced glasses were examined by X-ray absorption spectroscopy (XAS) and Raman spectroscopy. XAS spectra demonstrated that the oxidation numbers of Zr and Er ions were +4 and +3, respectively. The chemical environment around both cations was six-fold coordination. In addition, Raman spectra demonstrated that the Zr4+ ions formed the Q4(Zr) structure, which caused the reduction of non-bridging oxygen. In case of the Er3+ ions, the formation of the Si-O-Er bonds was explained from the Raman study. The chemical durability of glass was determined from Na+ ions leaching values. In pH 7 solution, the leached Na+ ions reduced from 25.67% to 21.43% and from 22.50% to 20.49% as a function of concentration of ZrO2 (x = 5 mol% to 15 mol%) and Er2O3 (y = 0.5 mol% to 1.5 mol%), respectively. As the results, the chemical durability of the ZrO2-containing and Er2O3-containing glasses were significantly improved due to charge compensated mechanism and enhancing network rigidity by increasing cation field strength. Moreover, the micro-hardness (580 HV to 837 HV) and density (2.54 g⸳cm-3 to 2.82 g⸳cm-3) also displayed an increased tendency with larger concentration of ZrO2 and Er2O3.
Downloads
References
N. Mascaraque, M. Bauchy, and M. Smedskjaer, "Correlating the network topology of oxide glasses with their chemical durability," The Journal of Physical Chemistry B, vol. 121, no. 5, pp. 1139-1147, 2017.
M. D. Bardi, H. Hutter, M. Schreiner, and R. Bertoncello, "Potash-lime-silica glass: protection from weathering," Heritage Science, vol. 3, no. 22, pp. 1-9, 2012.
W. Deng, J. Cheng, P. Tian, and M. Wang, "Chemical durability and weathering resistance of canasite based glass and glass-ceramics," Journal of Non-Crystalline Solids, vol. 358, no. 21, pp. 2847-2854, 2012.
E. Meechoowas, P. Jampeerung, K. Tapasa, U. Pantulap, and T. Jitwatcharakomol, "Glass batch modification to improve the weathering resistance in soda-lime silicate glass," Key Engineering Materials, vol. 798, pp. 206-211, 2019.
A. K. Varshneya, and J. C. Mauro, Fundamentals of Inorganic Glasses, Amsterdam: Elsevier Inc, 2019.
R. Makhlouk, Z. Chabbou, Y. Er-rouissi, M. Taibi, and S. Aqdim, "Chemical durability, Properties and structural approach of the glass series xFe2O3-(45-x) PbO-55P2O5 (with 0 ≤ x ≤ 20; mol%)," New Journal of Glass and Ceramics, vol. 13, pp. 1-16, 2023.
J. Zhao, Y. Wang, J. Kang, Y. Qu, G. Khater, S. Lia, Q. Shia, and Y. Yue, "Effect of SnO2 on the structure and chemical durability of the glass prepared by red mud," Journal of Non-Crystalline Solids, vol. 509, pp. 54-59, 2019.
S. Li, Y. Lu, Y. Qua, Y. Xu, L. Ming, Z. Song, and Y. Yue, "Influences of ZnO on the chemical durability and thermal stability of calcium iron phosphate glasses," Journal of Non-Crystalline Solids, vol. 498, pp. 228-235, 2018.
L. Xiongwei, L. Mei, W. Mitang, L. Zhaogang, H. Yanhong, and T. Junhu, "Effects of neodymium and gadolinium on weathering resistance of ZnO-B2O3-SiO2 glass," Journal of Rare Earths, vol. 32, no. 9, pp. 874-878, 2014.
T. Poungkaew, M. Jaimasith, P. Leowkijsiri, and W. Thiemsorn, "Industrial soda-lime-silica sheet glass hardened by zirconia-reinforced inorganic coating," TNI Journal of Engineering and Technology, vol. 2, no. 1, pp. 6-10, 2014.
J. Fisher, P. James, and J. Parker, "Soda lime zirconia silicate glasses as prospective hosts for zirconia-containing radioactive wastes," Journal of Non-Crystalline Solids, vol. 351, no. 8-9, pp. 623-631, 2005.
I. Hussain, E. K. Barimah, Y. Iqbal, G. Jose, A. Zeb, and R. Muhammad, "Mechanical and optical properties of ZrO2 doped silicate glass ceramics," Silicon, vol. 13, pp. 877-883, 2021.
M. Lobanova, A. Ledieu, P. Barboux, F. Devreux, O. Spalla, and J. Lambard, "Effect of ZrO2 on the glass durability Materials," Research Society, vol. 713, no. 151, pp. 1-9, 2002.
C. Cailleteau, F. Algeli, F. Devreux, S. Gin, J. Jestin, P. Jollivet, and O. Spalla, "Insight into silicate-glass corrosion mechanisms," Nature Materials, vol. 7, pp. 978-983, 2008.
A. Quintas, D. Caurant, O. Majerus, P. Loiseau, T. Charpentier, and J. Dussossoy, "ZrO2 addition in soda-lime aluminoborosilicate glasses containing rare earths: Impact on the network structure," Journal of Alloys and Compounds, vol. 714, pp. 47-62, 2017.
L. Cormier, O. Dargaud, G. Calas, C. Jousseaume, and S. Papin, "Zr environment and nucleation role in aluminosilicate glasses," Materials Chemistry and Physics, vol. 152, pp. 41-47, 2015.
P. Jollivet, G. Calas, L. Galoisy, F. Angeli, B. Bergeron, S. Gin, M. Ruffoni, and N. Trcera, "An enhanced resolution of the structural environment of zirconium in borosilicate glasses," Journal of Non-Crystalline Solids, vol. 381, pp. 40-47, 2013.
X. Lu, L. Deng, S. Kerisit, and J. Du, "Structural role of ZrO2 and its impact on properties of boroaluminosilicate nuclear waste glasses," npj Materials Degradation, vol. 2, no. 19, pp. 1-10, 2018.
F. Angeli, F. T. Charpentier, D. Ligny, and C. Cailleteau, "Boron speciation in soda lime borosilicate glasses containing zirconium," Journal of the American Ceramic Society, vol. 93, no. 9, pp. 2693-2704, 2010.
Z. Wang, and L. Cheng, "Effects of doping CeO2/TiO2 on structure and properties of silicate glass," Journal of Alloys and Compounds, vol. 597, pp. 167-174, 2014.
F. Lofaj, R. Satet, M. Hoffmann, F. Dorčáková, and A. R. D. A. López, "Rheological properties of the rare-earth doped glasses," Key Engineering Materials, Vols. 264-268, pp. 1867-1870, 2004.
A. V. Deepa, P. Vinothkumar, K. S. Moorthy, P. Muralimanohar, M. Mohapatra, S. Praveenkumar, and P. Murugasen, "Optical, electrical, mechanical properties of Pr3+ and Yb3+ doped phosphate glasses," Optical and Quantum Electronics, vol. 52, no. 11, pp. 1-28, 2020.
F. Lofaj, P. Hvizdos, F. Dorcˇa´kova´, R. Satet, M. J. Hoffmann, and A. R. D. Arellano-Lo´pez, "Indentation moduli and micro-hardness of RE-Si-Mg-O-N glasses (RE=Sc, Y, La, Sm, Yb and Lu) with different nitrogen content," Materials Science and Engineering, vol. 357, no. 1-2, pp. 181-187, 2003.
P. P. Puga, P. Danyliuk, A. I. Gomonai, H. V. Rizak, I. M. Rizak, V. M. Rizak, G. D. Puga, L. Kvetková, and M. M. Byrov, "Raman scattering in glassy Li2B4O7 doped with Er2O3," Ukrainian Journal of Physical Optics, vol. 19, no. 4, pp. 211-219, 2018.
O. Majérus, D. Caurant, A. Quintas, J. Dussossoy, I. Bardez, and P. Loiseau, "Effect of boron oxide addition on the Nd3+ environment in a Nd-rich soda-lime aluminoborosilicate glass," Journal of Non-Crystalline Solids, vol. 357, no. 14, pp. 2744-2751, 2011.
M. Wang, J. Cheng, M. Li, and F. He, "Raman spectra of soda–lime–silicate glass doped with rare earth," Physica B, vol. 406, no. 20, pp. 3865-3869, 2011.
D. Caurant, P. Loiseau, O. Majérus, V. A. Chevaldonnet, I. Bardez, and A. Quintas, Glasses, glass-ceramics and ceramics for immobilization of highly radioactive nuclear, New York: Nova Science Publishers, Inc., 2009.
A. Quintas, D. Caurant, O. Majerus, P. Loiseau, T. Charpentier, and J. Dussossoy, "ZrO2 addition in soda-lime aluminoborosilicate glasses containing rare earths: Impact on rare earths environment and crystallization," Journal of Alloys and Compounds, vol. 719, pp. 383-397, 2017.
M. Wang, J. Cheng, Q. Liu, P. Tian, and M. Li, "The effect of light rare earths on the chemical durability and weathering of Na2O–CaO–SiO2 glasses," Journal of Nuclear Materials, vol. 400, no. 2, pp. 107-111, 2010.
R. M. Almeida, and L. F. Santos, "Raman spectroscopy of glasses," in Modern Glass Characterization, Ed. Hoboken, Wiley-American Ceramic Society, 2015, pp. 74-106.
M. F. Dilmore, D. E. Clark, and L. L. Hence, "Chemical durability of Na2O‐K2O‐CaO‐SiO2 glasses," Journal of the American Ceramic Society, vol. 61, no. 9-10, pp. 439-443, 2006.
D. R. Neuville, "Viscosity, structure and mixing in (Ca, Na) silicate melts," Chemical Geology, vol. 229, no. 1-3, pp. 28-41, 2006.
T. Furukawa, K. E. Fox, and W. B. White, "Raman spectroscopic investigation of the structure of silicate glasses. III. Raman intensities and structural units in sodium silicate glasses," The Journal of Chemical Physics, vol. 75, pp. 3226-3237, 1981.
K. Fukumi, J. Hayakawa, and T. Komiyama, "Intensity of Raman band in silicate glasses," Journal of Non-Crystalline Solids, vol. 119, no. 3, pp. 297-302, 1990.
A. K. Yadav, and P. Singh, "A review of the structures of oxide glasses by Raman spectroscopy," RSC Advances, vol. 5, pp. 67583-67609, 2015.
M. Ficheux, E. Burov, G. Aquilanti, N. Trcera, V. Montouillout, and L. Cormier, "Structural evolution of high zirconia alumino-silicate glasses," Journal of Non-Crystalline Solids, vol. 539, pp. 1-11, 2020.
J. S. McCloy, J. Marcial, D. Patil, M. Saleh, M. Ahmadzadeh, H. Chen, J. V. Crum, B. J. Riley, H. Kamat, A. Bréhault, A. Goel, K. E. Barnsley, J. V. Hanna, P. Rajbhandari, and C. L. Corkhill, "Glass structure and crystallization in boro-alumino-silicate glasses containing rare earth and transition metal cations," MRS Advances , vol. 4, p. 1029-1043, 2019.
N. Chouard, D. Caurant, O. Majérus, J. L. Dussossoy, S. Klimin, D. Pytalev, R. Baddour-Hadjean, and J. P. Pereira-Ramos, "Effect of MoO3, Nd2O3, and RuO2 on the crystallization of soda–lime aluminoborosilicate glasses," Journal of Materials Science, vol. 50, p. 219-241, 2015.
G. E. Brown, F. Farges, and G. Calas, "X-ray scattering and X-ray spectroscopy studies of silicate melts," Reviews in Mineralogy and Geochemistry, vol. 32, pp. 317-410, 1995.
J. Du, and A. N. Cormack, "The structure of erbium doped sodium silicate glasses," Journal of Non-Crystalline Solids, vol. 351, no. 27-29, pp. 2263-2276, 2005.
L. Robinet, D. Neff, A. Bouquillon, S. P. Camagna, and A. V. Carron, "Raman spectroscopy, a non-destructive solution to the study of glass and its alteration," in ICOM 15th triennial conference, New Delhi, 2008.
A. J. G. Ellison, and P. C. Hess, "Lanthanides in silicate glasses: A vibrational spectroscopic study," Journal of Geophysical Research: Solid Earth, vol. 95, no. B10, pp. 15,717-15,726, 1990.
A. J. G. Ellison, and P. C. Hess, "Vibrational spectra of high-silica glasses of the system K2O-SiO2-La2O3," Journal of Non-Crystalline Solids, vol. 127, no. 3, pp. 247-258, 1991.
R. B. Greegor, K. Y. Blohowiak, J. H. Osborne, K. A. Krienke, and J. T. Cherian, "X-ray spectroscopic investigation of the Zr-site in thin film sol-gel surface preparations," Journal of Sol-Gel Science and Technology, vol. 20, pp. 35-50, 2001.
L. Cormier, O. Dargaud, G. Calas, C. Jousseaume, S. Papin, N. Trcera, and A. Cognigni, "Zr environment in alumino-silicate glasses Zr environment and nucleation role in alumino-silicate glasses," Materials Chemistry and Physics, vol. 152, pp. 41-47, 2015.
K. Samkongngam, "Spectroscopic studies of transition metal ions in silicate glass," Bangkok, 2019.
W. Kaewwiset, K. Thamaphat, J. Kaewkhao, and P. Limsuwan, "Er3+-doped soda-lime silicate glass: artificial pink gemstone," American Journal of Applied Sciences, vol. 11, pp. 1769-1775, 2012.
Y. Lin, M. M. Smedskjaer, and J. C. Mauro, "Structure, properties, and fabrication of calcium aluminate-based glasses," The International Journal of Applied Glass Science, vol. 10, pp. 488-501, 2019.
K. Januchta, M. Bauchy, R. E. Youngman, S. J. Rzoska, M. Bockowski, and M. M. Smedskjaer, "Modifier field strength effects on densification behavior and mechanical properties of alkali aluminoborate glasses," Physical Review Materials, vol. 1, no. 6, pp. 1-12, 2017.
B. C. Bunker, "Molecular mechanisms for corrosion of silica and silicate glasses," Journal of Non-Crystalline Solids, vol. 179, pp. 300-308, 1994.
M. Kim, C. L. Corkhill, N. C. Hyatt, and J. Heo, "Development, characterization and dissolution behavior of calcium-aluminoborate glass wasteforms to immobilize rare-earth oxides," Scientific Reports, vol. 8, pp. 1-8, 2018.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












