Atmospheric hydrochloric and nitric acid leaching of a limonite ore from the Wolo mine area, Southeast Sulawesi, Indonesia
DOI:
https://doi.org/10.55713/jmmm.v34i1.1875Keywords:
Limonite ore, X-ray diffraction, Goethite, Hydrochloric acid, Nitric acidAbstract
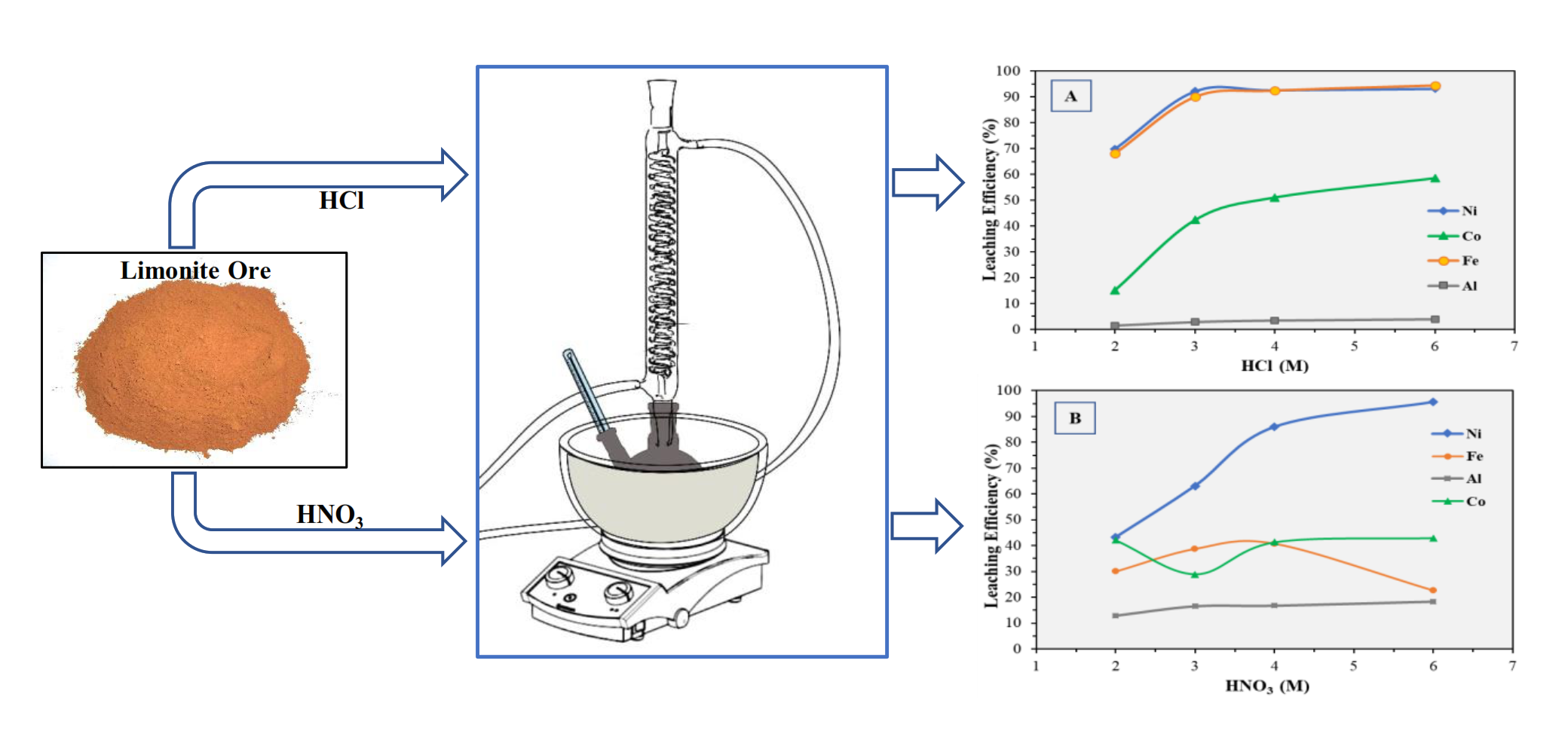
The atmospheric acid leaching studies of a limonite ore sample from the Wolo mine area, Southeast Sulawesi, Indonesia, have been performed using hydrochloric acid (HCl) and nitric acid (HNO3). The objectives of these studies were to compare the leaching degree of metals (Ni, Co, Fe, and Al) and to analyze the dissolution behavior of minerals under different acid concentrations. Mineralogical characterization of the ore sample was conducted using optical microscopy, scanning electron microscopy (SEM), and X-ray diffraction, whereas chemical composition was determined by X-ray fluorescence (XRF) spectrometry and atomic absorption spectroscopy (AAS), respectively. An atmospheric leaching test was done with the variables of acid concentration, leaching duration of 90 min, and leaching temperature of 100℃. Limonite ore samples contain goethite, gibbsite, talc, quartz, and lizardite. It was revealed that as much as 92.22% of Ni and 90.14% of Fe could be leached using 3 M HCl, whereas only 63.14% of Ni and 38.74% of Fe could be extracted from limonite ore using 3 M HNO3. The higher leaching degree of Fe in HCl indicates low selectivity with Ni, which might contaminate pregnant leach solution (PLS), leading to further complications in the purification process. Results of the leaching experiment show that goethite was more easily dissolved in HCl than in HNO3.
Downloads
References
Nickel Institute, “About Nickel”. https://nickelinstitute.org/
about-nickel/. Accessed on 17 August 2023.
C. R. M. Butt, and D. Cluzel, “Nickel laterite ore deposits: weathered serpentinites,” Elements, vol. 9, pp. 123-128, 2013.
U.S. Geological Survey, “Mineral commodity summaries”: U.S. Geological Survey, 210 p, 2023. https://doi.org/10.3133/
mcs2023US, Accessed on 15 October 2023.
A. Ito, T. Otake, A. Maulana, K. Sanematsu, Sufriadin, and T. Sato, “Geochemical constraints on the mobilization on the mobilization of Ni and critical metals in laterite deposits, Sulawesi, Indonesia: A mass-balance approach”, Resources Geology, vol.71, pp. 255-282, 2021.
V. I. Lakshmanan, R. Sridhar, J. Chen, and M. A. Halim, “Development of mixed-chloride hydrometallurgical processes for the recovery of value metals from various resources”, Transaction of Indian Institute of Metallurgy, vol. 69, pp. 39-50, 2016.
Sufriadin, S. Widodo, I. Nur, A. Ilyas, and M. Y. Ashari, “Extraction of nickel and cobalt from sulawesi limonite ore in nitric acid solution at atmospheric pressure”, IOP Conference Series.: Material Science and. Engineering, vol. 875, p. 012053, 2020.
K. Liu, Q. Y. Chen, H. P. Hu, and Y. Yin, “Characterization and leaching behavior of lizardite in Yunjiang laterite ore”, Applied Clay Science, vol. 47, pp. 311-316, 2010.
Z. Zhou, B. Ma, C. Wang, Y. Chen, W. Zhang, and K. Huang, “Enrichment of scandium and aluminum from limonitic laterite during the nitric acid pressure leaching process”, Hydrometallurgy, vol. 208, 2022.
J. Li, Y. Yang, Y. Wen, W. Liu, Y. Chu, R. Wang, and Z. Xu, “Leaching kinetics and mechanism of laterite with NH4C‒HCl solution”, Minerals, vol.10, 2020.
S. Stanković, S. Stopić, M. Sokić, B. Marković, and B. Friedrich, “Review of the past, present, and future of the hydrometallurgical production of nickel and cobalt from lateritic ores”. Metallurgical and Material Engineering, vol.26, no. 2, pp. 199-208, 2020.
M. A. R. Onal, and Y. A.Topkaya, “Pressure acid leaching of Çaldağ lateritic nickel ore: An alternative to heap leaching”, Hydrometallurgy, vol.142, pp. 98-107, 2014.
J. Li, D. Xiong, H. Chen, R. Wang, and Y. Liang, “Physico-chemical factors affecting leaching of laterite ore in hydrochloric acid”, Hydrometallurgy, vol.129-130, pp.14-18, 2012.
B. Ma, W. Yang, B. Yang, C. Wang, Y. Chen, and Y. Zhang, “Pilot-scale plant study on the innovative nitric acid pressure leaching technology for laterite ores”, Hydrometallurgy, vol. 155, pp. 88-94, 2015.
M. Rao, J. Chen, T. Zhang, M. Hu, J. You, and J. Luo, “Atmospheric acid leaching of powdery Ni-Co-Fe alloy derived from reductive roasting of limonitic laterite ore and recovery of battery grade iron phosphate”, Hydrometallurgy, vol. 218, pp. 1-10, 2023.
I. M. Ugwu, and D. M. Sherman, “The solubility of goethite with structurally incorporated nickel and cobalt: Implication for laterites”, Chemical Geology, vol. 518, pp. 1-8, 2019.
Y. Changa, K. Zhao, and B. Pešić, “Selective leaching of nickel from pre-reduced limonitic laterite under moderate HPAL conditions- Part I: Dissolution”, Journal of Mining and Metallurgy, Section B. Metallurgy, vol.52, pp. 127-134, 2016.
A. Garces-Granda, G.T. Lapidus, and O.J. Restrepo-Baena, “Effect of a thermal pretreatment on dissolution kinetics of a limonitic laterite ore in chloride media”, Hydrometalurgy, vol. 196, p. 105428, 2020.
R.G. McDonald, and B.I. Whittington, “Atmospheric acid leaching of nickel laterites review. Part II. Chloride and bio-technologies”, Hydrometallurgy, vol. 91, pp. 56-69, 2008.
F. He, B. Ma, C. Wang, and Y. Chen, “Mineral evolution and porous kinetics of limonitic laterite during nitric acid pressure leaching”, Mineral Engineering, vol.181, p. 107544, 2022.
O. Levenspiel, “Chemical reaction engineering” 3rd ed. John Wiley & Sons, Inc. New York, 668 p. 1999.
F. He, B. Ma, C. Wang, Y. Zuo, and Y. Chen, “Dissolution behavior and porous kinetics of limonitic laterite during nitric acid atmospheric leaching”, Mineral Engineering, vol.185, p. 107671, 2022.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












