Elucidating the corrosion of carbon steel in hybrid monoethanolamine solutions containing methanol or n-methyl-2-pyrrolidone
DOI:
https://doi.org/10.55713/jmmm.v35i2.2157Keywords:
CO2 absorption, Carbon steel corrosion, CO2 capture, Hybrid MEA solutionAbstract
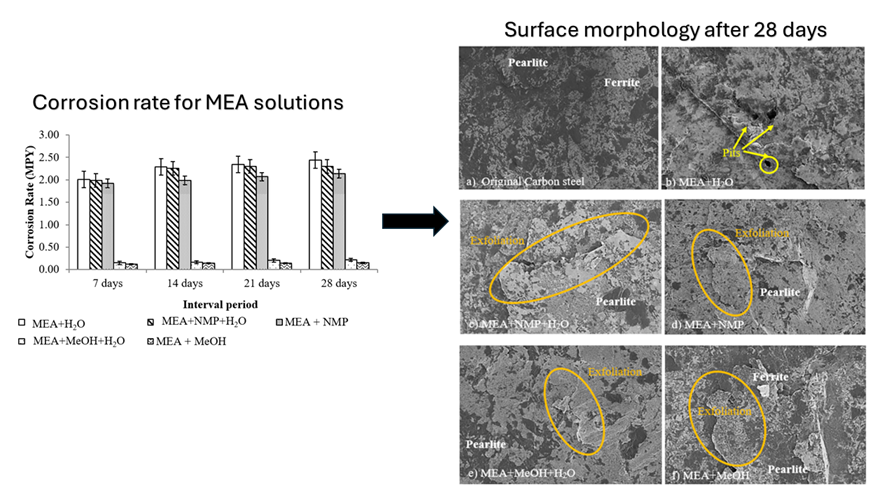
Corrosion has been a persistent issue in carbon dioxide (CO2) absorption unit that needs to be resolved in order to prolong the life span of the absorber unit. In this regard, understanding the corrosion behaviour, particularly in hybrid monoethanolamine (MEA) solutions, is crucial. This study aims to give detail information on the role of different process parameters, encompassing types of amine solution, temperature, and CO2 loading on the corrosion behaviour of carbon steel in system containing hybrid solution of monoethanolamine (MEA) and methanol (MeOH) or N-methyl-2-pyrrolidone (NMP). Here, the corrosion extent of the carbon steel was evaluated using gravimetric experiments. The results indicated that the corrosion rate of carbon steels, when submerged in various amine solutions, increased with higher solution temperatures and the presence of CO2. In line with the Raman spectroscopy results and the surface morphology analysis, results also showed that the carbon steel coupons immersed in MEA+MeOH had the lowest corrosion rate.
Downloads
References
N. Van Nguyen, V. Pirouzfar, H. Soheilinezhad, and C.-H. Su, "Optimizing the CO2 capture and removal process to recover energy and CO2 from the flue gas of boilers and gas turbines outlet with considering the techno-economic analysis," Energy, vol. 291, p. 130281, 2024. DOI: https://doi.org/10.1016/j.energy.2024.130281
U. Khan, C. C. Ogbaga, O.-A. O. Abiodun, A. A. Adeleke, P. P. Ikubanni, P. U. Okoye, and J. A. Okolie, "Assessing absorption-based CO2 capture: Research progress and techno-economic assessment overview," Carbon Capture Science & Technology, vol. 8, p. 100125, 2023. DOI: https://doi.org/10.1016/j.ccst.2023.100125
Z. L. Ooi, P. Y. Tan, L. S. Tan, and S. P. Yeap, "Amine-based solvent for CO2 absorption and its impact on carbon steel corrosion: A perspective review," Chinese Journal of Chemical Engineering, vol. 28, pp. 1357-1367, 2020. DOI: https://doi.org/10.1016/j.cjche.2020.02.029
S. C. Tiwari, A. Bhardwaj, K. D. P. Nigam, K. K. Pant, and S. Upadhyayula, "A strategy of development and selection of absorbent for efficient CO2 capture: An overview of properties and performance," Process Safety and Environmental Protection, vol. 163, pp. 244-273, 2022. DOI: https://doi.org/10.1016/j.psep.2022.05.025
B. Dutcher, M. Fan, and A. G. Russell, "Amine-based CO2 capture technology development from the beginning of 2013—A Review," ACS Applied Materials & Interfaces, vol. 7, pp. 2137-2148, 2015. DOI: https://doi.org/10.1021/am507465f
A. Sánchez-Bautista, E. M. Palmero, A. J. Moya, D. Gómez-Díaz, and M. D. La Rubia, "Characterization of alkanolamine blends for carbon dioxide absorption, corrosion and regeneration studies," Sustainability, vol. 13, p. 4011, 2021. DOI: https://doi.org/10.3390/su13074011
M. Javidi, H. Karimi Abadeh, F. Namazi, H. R. Yazdanpanah, and N. Shirvani Shiri, "A new perspective on the corrosion of carbon steels in H2SO4 acid environments: Statistical analysis of corrosion mechanisms by response surface modeling," Materials Chemistry and Physics, vol. 312, p. 128608, 2024. DOI: https://doi.org/10.1016/j.matchemphys.2023.128608
D. Dwivedi, K. Lepková, and T. Becker, "Carbon steel corrosion: a review of key surface properties and characterization methods," RSC Advances, vol. 7, pp. 4580-4610, 2017. DOI: https://doi.org/10.1039/C6RA25094G
K. Liao, M. Qin, N. Yang, G. He, S. Zhao, and S. Zhang, "Corrosion main control factors and corrosion degree prediction charts in H2S and CO2 coexisting associated gas pipelines," Materials Chemistry and Physics, vol. 292, p. 126838, 2022. DOI: https://doi.org/10.1016/j.matchemphys.2022.126838
P. Gunasekaran, A. Veawab, and A. Aroonwilas, "Corrosivity of Amine-Based Absorbents for CO2 Capture," Energy Procedia, vol. 114, pp. 2047-2054, 2017. DOI: https://doi.org/10.1016/j.egypro.2017.03.1339
F. Stergioudi, A. Baxevani, C. Florou, N. Michailidis, E. Nessi, A. I. Papadopoulos, and P. Seferlis, "Corrosion Behavior of stainless steels in CO2 absorption process using aqueous solution of monoethanolamine (MEA)," Corrosion and Materials Degradation, vol. 3, pp. 422-438, 2022. DOI: https://doi.org/10.3390/cmd3030025
N. S. Radhi, Z. Al-Khafaji, B. M. Mareai, S. Radhi, and A. M. Alsaegh, "Reducing oil pipes corrosion by (Zn-Ni) alloy coating on low carbon steel substrate by sustainable process," Journal of Engineering Science and Technology, vol. 18, pp. 1624-1638, 2023.
R. R. Wanderley, D. D. D. Pinto, and H. K. Knuutila, "From hybrid solvents to water-lean solvents – A critical and historical review," Separation and Purification Technology, vol. 260, p. 118193, 2021. DOI: https://doi.org/10.1016/j.seppur.2020.118193
J. Gervasi, L. Dubois, and D. Thomas, "Screening tests of new hybrid solvents for the post-combustion CO2 capture processby chemical absorption," Energy Procedia, vol. 63, pp. 1854-1862, 2014. DOI: https://doi.org/10.1016/j.egypro.2014.11.193
L. S. Tan, A. M. Shariff, K. K. Lau, and M. A. Bustam, "Impact of high pressure on high concentration carbon dioxide capture from natural gas by monoethanolamine/N-methyl-2-pyrrolidone solvent in absorption packed column," International Journal of Greenhouse Gas Control, vol. 34, pp. 25-30, 2015. DOI: https://doi.org/10.1016/j.ijggc.2014.12.020
H. Rashidi, and S. Sahraie, "Enhancing carbon dioxide absorption performance using the hybrid solvent: Diethanolamine-methanol," Energy, vol. 221, p. 119799, 2021. DOI: https://doi.org/10.1016/j.energy.2021.119799
Y. Xiang, W. Xie, S. Ni, and X. He, "Comparative study of A106 steel corrosion in fresh and dirty MEA solutions during the CO2 capture process: Effect of NO3−," Corrosion Science, vol. 167, p. 108521, 2020. DOI: https://doi.org/10.1016/j.corsci.2020.108521
N. Wang, D. Wang, A. Krook-Riekkola, and X. Ji, "MEA-based CO2 capture: a study focuses on MEA concentrations and process parameters," Frontiers in Energy Research, vol. 11, 2023. DOI: https://doi.org/10.3389/fenrg.2023.1230743
K. O. Oparaodu and G. C. Okpokwasili, "Comparison of percentage weight loss and corrosion rate trends in different metal coupons from two soil environments," International Journal of Environmental Bioremediation & Biodegradation, vol. 2, pp. 243-249, 2014.
Y. Wang, M. Fang, T. Wang, J. Gao, Y. Huang, S. Li, X. Lu, Y. Sun, and F. Zhang, "Corrosion performance of carbon/ stainless steel in amine-based solvents under different conditions for CO2 chemical absorption process," Greenhouse Gases: Science and Technology, vol. 14, no. 1, p. 2250, 2023. DOI: https://doi.org/10.1002/ghg.2250
M. Hasib-ur-Rahman, H. Bouteldja, P. Fongarland, M. Siaj, and F. ç. Larachi, "Corrosion behavior of carbon steel in alkanolamine/ room-temperature ionic liquid based CO2 capture systems," Industrial & Engineering Chemistry Research, vol. 51, pp. 8711-8718, 2012. DOI: https://doi.org/10.1021/ie2019849
P. Usubharatana, and P. Tontiwachwuthikul, "Enhancement factor and kinetics of CO2 capture by MEA-methanol hybrid solvents," Energy Procedia, vol. 1, pp. 95-102, 2009. DOI: https://doi.org/10.1016/j.egypro.2009.01.015
American Society for Testing and Materials (ASTM), "Standard practice for preparing, cleaning, and evaluating corrosion test specimens," ed. West Conshohocken, USA, 2014.
L. Yang, "1 - Introduction," in Techniques for Corrosion Monitoring (Second Edition), L. Yang, Ed., ed: Woodhead Publishing, 2021, pp. 1-5. DOI: https://doi.org/10.1016/B978-0-08-103003-5.00001-1
P. Pedeferri, Corrosion science and engineering: Springer, 2018. DOI: https://doi.org/10.1007/978-3-319-97625-9
G. N. Haidemenopoulos, H. Kamoutsi, K. Polychronopoulou, P. Papageorgiou, I. Altanis, P. Dimitriadis, and M. Stiakakis, "Investigation of stress-oriented hydrogen-induced cracking (SOHIC) in an amine absorber column of an oil refinery," Metals, vol. 8, p. 663, 2018. DOI: https://doi.org/10.3390/met8090663
Y. Xiang, Z. Wang, Z. Li, and W. D. Ni, "Effect of exposure time on the corrosion rates of X70 steel in supercritical CO2/ SO2/O2/H2O environments," Corrosion, vol. 69, pp. 251-258, 2013. DOI: https://doi.org/10.5006/0769
K. Vishwanath, B. Bhushan, S. S. Singh, and K. Mondal, "Atmospheric corrosion behavior of ferritic-pearlitic, spheroidized, and bainitic microstructures of a mild steel," Materials Today Communications, vol. 40, p. 109544, 2024. DOI: https://doi.org/10.1016/j.mtcomm.2024.109544
E. Khamme and R. Sakdanuphab, "Study of corrosion properties of carbon steel, 304 and 316L stainless steels in sulfuric acid and their degradation products," Journal of Metals, Materials and Minerals, vol. 33, p. 1672, 2023. DOI: https://doi.org/10.55713/jmmm.v33i4.1672
L. Yarris, "Alcohol and water don't mix," University of California, 2003.
A. Veawab, P. Tontiwachwuthikul, and A. Chakma, "Corrosion behavior of carbon steel in the CO2 absorption process using aqueous amine solutions," Industrial & Engineering Chemistry Research, vol. 38, pp. 3917-3924, 1999. DOI: https://doi.org/10.1021/ie9901630
P. Y. Tan, L. S. Tan, S. P. Yeap, K. W. Kow, A. M. Shariff, and M. K. Wong, "Corrosion of carbon steel in hybrid n-methyl-2-pyrrolidone - monoethanolamine solutions for carbon dioxide absorption unit application," IOP Conference Series: Materials Science and Engineering, vol. 736, p. 022086, 2020. DOI: https://doi.org/10.1088/1757-899X/736/2/022086
J. Yang, "Corrosion behavior of carbon steel in carbonated MDEA-MEA aqueous solutions," IOP Conference Series: Earth and Environmental Science, vol. 446, p. 032087, 2020. DOI: https://doi.org/10.1088/1755-1315/446/3/032087
K. Tuutti, "Service life of structures with regard to corrosion of embedded steel," ACI Symposium Publication, vol. 65, 1980.
V. Živica, "Significance and influence of the ambient temperature as a rate factor of steel reinforcement corrosion," Bulletin of Materials Science, vol. 25, pp. 375-379, 2002. DOI: https://doi.org/10.1007/BF02708013
P. Schiessl, and M. Raupach, "26 - Chloride-induced corrosion of steel in concrete - investigations with a concrete corrosion cell," in The Life of Structures, G. S. T. Armer, J. L. Clarke, and F. K. Garas, Eds., ed: Butterworth-Heinemann, 1989, pp. 226-233. DOI: https://doi.org/10.1016/B978-0-408-04245-1.50030-3
A. Zaher, A. Chaouiki, R. Salghi, A. Boukhraz, B. Bourkhiss, and M. Ouhssine, "Inhibition of mild steel corrosion in 1m hydrochloric medium by the methanolic extract of Ammi visnaga L. Lam Seeds," International Journal of Corrosion, vol. 2020, p. 9764206, 2020. DOI: https://doi.org/10.1155/2020/9764206
Z. Liang, W. Rongwong, H. Liu, K. Fu, H. Gao, F. Cao, R. Zhang, T. Sema, A. Henni, K. Sumon, D. Nath, D. Gelowitz, W. Srisang, C. Saiwan, A. Benamor, M. Al-Marri, H. Shi, T. Supap, C. Chan, Q. Zhou, M. Abu-Zahra, M. Wilson, W. Olson, R. Idem, and P. Tontiwachwuthikul, "Recent progress and new developments in post-combustion carbon-capture technology with amine based solvents," International Journal of Greenhouse Gas Control, vol. 40, pp. 26-54, 2015. DOI: https://doi.org/10.1016/j.ijggc.2015.06.017
L. Xu, D. Mei, and G. Henkelman, "Adaptive kinetic Monte Carlo simulation of methanol decomposition on Cu(100)," Journal of Chemical Physics, vol. 131, pp. 244-520, 2009. DOI: https://doi.org/10.1063/1.3281688
Downloads
Published
How to Cite
License
Copyright (c) 2025 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












