High-binding affinity nanobody against SARS-CoV-2 XBB.1.5: Computational-based protein engineering
DOI:
https://doi.org/10.55713/jmmm.v35i2.2184Keywords:
Computational-based Protein Engineering, HDOCK, Nanobody, SARS-CoV-2 XBB.1.5Abstract
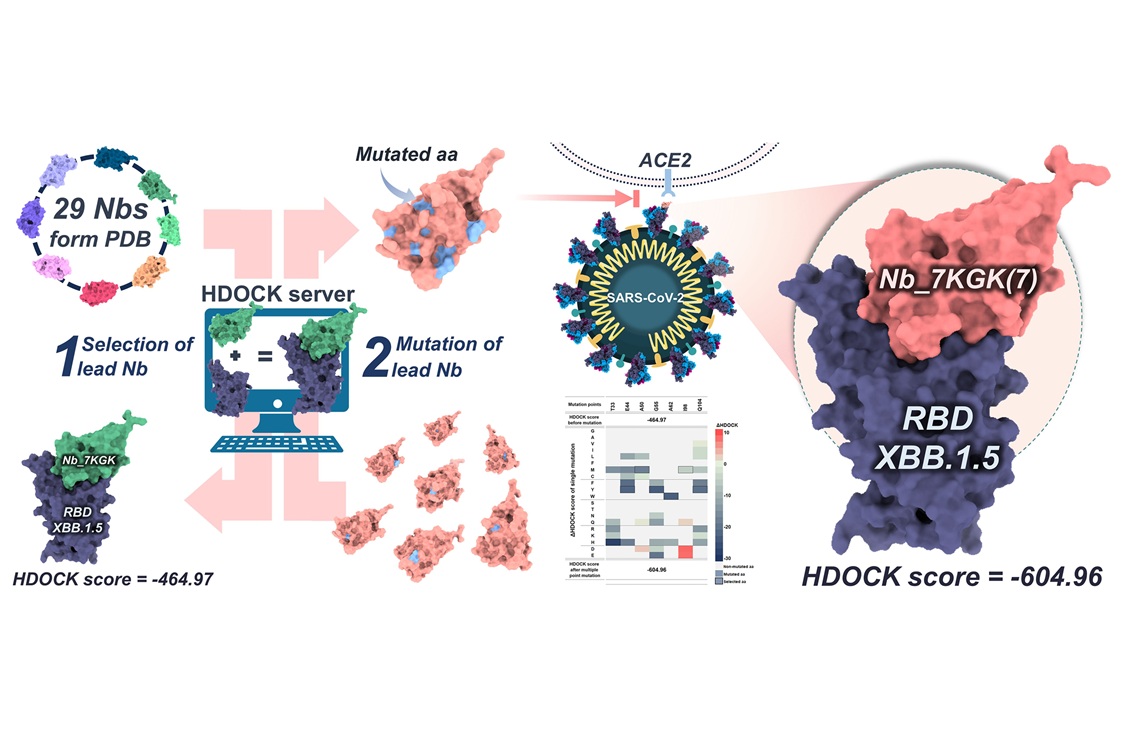
The emergence of the SARS-CoV-2 variant XBB.1.5 has triggered a global health crisis by enhancing viral entry into cells via its spike protein. This study addresses the urgent need to develop neutralizing nanobodies (Nbs) to counteract the SARS-CoV-2 virus. Our aim was to identify a lead Nb and enhance its binding affinity to the receptor-binding motif (RBM) on the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein (S-protein) using computational methods. A total of 29 Nbs were screened against the XBB.1.5 RBD using the HDOCK server to select a lead Nb. This investigation revealed that Nb_7KGK exhibited the highest binding affinity. Subsequently, unfavorable residues of Nb_7KGK were mutated to further enhance binding affinity. As expected, aromatic residues (tyrosine, tryptophan, histidine) were primarily mutated to improve binding affinity, resulting in a new Nb variant named Nb_7KGK(7), with heightened affinity. The engineered Nb_7KGK(7) demonstrated improved chemical interactions with the RBD. Predicted physicochemical properties, such as pI value, total charge, Clashscore, and MolProbity score of the engineered Nb, were also improved. This study highlights the potential of computational design as a preliminary step toward developing effective Nbs against the emerging SARS-CoV-2 XBB.1.5 variant.
Downloads
References
T. Sharma, B. Gerstman, and P. Chapagain, “Distinctive features of the XBB.1.5 and XBB.1.16 spike protein receptor-binding domains and their roles in conformational changes and angiotensin-converting enzyme 2 binding,” International Journal of Molecular Sciences, vol. 24, no. 16, p. 12586, 2023. DOI: https://doi.org/10.3390/ijms241612586
K. Uriu, J. Ito, J. Zahradnik, S. Fujita, Y. Kosugi, G. Schreiber, and K. Sato, “Enhanced transmissibility, infectivity, and immune resistance of the SARS-CoV-2 omicron XBB.1.5 variant,” The Lancet Infectious Diseases, vol. 23, no. 3, pp. 280-281, 2023. DOI: https://doi.org/10.1016/S1473-3099(23)00051-8
C. Yue, W. Song, L. Wang, F. Jian, X. Chen, F. Gao, Z. Shen, Y. Wang, X. Wang, and Y. Cao, “Enhanced transmissibility of XBB.1.5 is contributed by both strong ACE2 binding and antibody evasion,” bioRxiv, p. 522427, 2023. DOI: https://doi.org/10.1101/2023.01.03.522427
D. V. Parums, “The XBB. 1.5 (‘Kraken’) subvariant of omicron SARS-CoV-2 and its rapid global spread,” Medical science monitor: international medical journal of experimental and clinical research, vol. 29, pp. e939580-1, 2023. DOI: https://doi.org/10.12659/MSM.939580
A. Sugano, H. Kataguchi, M. Ohta, Y. Someya, S. Kimura, Y. Maniwa, T. Tabata, and Y. Takaoka, “SARS-CoV-2 omicron XBB. 1.5 may be a variant that spreads more widely and faster than other variants,” bioRxiv, p. 524660, 2023. DOI: https://doi.org/10.1101/2023.01.18.524660
B. Ju, Q. Zhang, J. Ge, R. Wang, J. Sun, X. Ge, J. Yu, S. Shan, B. Zhou, and S. Song, “Human neutralizing antibodies elicited by SARS-CoV-2 infection,” Nature, vol. 584, no. 7819, pp. 115-119, 2020. DOI: https://doi.org/10.1038/s41586-020-2380-z
D. Ray, R. N. Quijano, and I. Andricioaei, “Point mutations in SARS-CoV-2 variants induce long-range dynamical perturbations in neutralizing antibodies,” Chemical science, vol. 13, no. 24, pp. 7224-7239, 2022. DOI: https://doi.org/10.1039/D2SC00534D
T. Zhou, L. Wang, J. Misasi, A. Pegu, Y. Zhang, D. R. Harris, A. S. Olia, C. A. Talana, E. S. Yang, M. Chen, M. Choe, W. Shi, I-T. Teng, A. Creanga, C. Jenkins, K. Leung, T. Liu, E.-S. D. Stancofski, T. Stephens, B. Zhang, Y. Tsybovsky, B. S. Graham, J. R. Mascola, N. J. Sullivan, P. D. Kwong, “Structural basis for potent antibody neutralization of SARS-CoV-2 variants including B.1.1.529,” Science, vol. 376, no. 6591, pp. eabn8897, 2022. DOI: https://doi.org/10.1126/science.abn8897
E. V. Capela, M. R. Aires-Barros, M. G. Freire, and A. M. Azevedo, “Monoclonal antibodies—addressing the challenges on the manufacturing processing of an advanced class of therapeutic agents,” Frontiers in Clinical Drug Research - Anti Infectives, vol. 4, no. 4, pp. 142, 2017. DOI: https://doi.org/10.2174/9781681084879117040007
C. Ackaert, N. Smiejkowska, C. Xavier, Y. G. Sterckx, S. Denies, B. Stijlemans, Y. Elkrim, N. Devoogdt, V. Caveliers, and T. Lahoutte, “Immunogenicity risk profile of nanobodies,” Frontiers in immunology, vol. 12, p. 632687, 2021. DOI: https://doi.org/10.3389/fimmu.2021.632687
J. Huo, A. Le Bas, R. R. Ruza, H. M. Duyvesteyn, H. Mikolajek, T. Malinauskas, T. K. Tan, P. Rijal, M. Dumoux, and P. N. Ward, “Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2,” Nature structural & molecular biology, vol. 27, no. 9, pp. 846-854, 2020. DOI: https://doi.org/10.1038/s41594-020-0469-6
G. Van Heeke, K. Allosery, V. De Brabandere, T. De Smedt, L. Detalle, and A. de Fougerolles, “Nanobodies® as inhaled biotherapeutics for lung diseases,” Pharmacology & Therapeutics, vol. 169, pp. 47-56, 2017. DOI: https://doi.org/10.1016/j.pharmthera.2016.06.012
Z. R. Crook, N. W. Nairn, and J. M. Olson, “Miniproteins as a powerful modality in drug development,” Trends in Biochemical Sciences, vol. 45, no. 4, pp. 332-346, 2020. DOI: https://doi.org/10.1016/j.tibs.2019.12.008
F. Y. Frejd, and K.-T. Kim, “Affibody molecules as engineered protein drugs,” Experimental & Molecular Medicine, vol. 49, no. 3, p. e306, 2017. DOI: https://doi.org/10.1038/emm.2017.35
E. L. Hesketh, C. Tiede, H. Adamson, T. L. Adams, M. J. Byrne, Y. Meshcheriakova, I. Kruse, M. J. McPherson, G. P. Lomonossoff, D. C. Tomlinson, and N. A. Ranson, “Affimer reagents as tools in diagnosing plant virus diseases,” Scientific Reports, vol. 9, no. 1, p. 7524, 2019. DOI: https://doi.org/10.1038/s41598-019-43945-6
O. Hantschel, “Monobodies as possible next-generation protein therapeutics - a perspective,” Swiss Medical Weekly, vol. 147, no. 47, p. 14545, 2017. DOI: https://doi.org/10.4414/smw.2017.14545
Y. Xiang, S. Nambulli, Z. Xiao, H. Liu, Z. Sang, W. P. Duprex, D. Schneidman-Duhovny, C. Zhang, and Y. Shi, “Versatile and multivalent nanobodies efficiently neutralize SARS-CoV-2,” Science, vol. 370, no. 6523, pp. 1479-1484, 2020. DOI: https://doi.org/10.1126/science.abe4747
M. Aksu, P. Kumar, T. Güttler, W. Taxer, K. Gregor, B. Mußil, O. Rymarenko, K. M. Stegmann, A. Dickmanns, S. Gerber, W. Reineking, C. Schulz, T. Henneck, A. Mohamed, G. Pohlmann, M. Ramazanoglu, K. Mese, U. Groß, T. Ben-Yedidia, O. Ovadia, D. W. Fischer, M. Kamensky, A. Reichman, W. Baumgärtner, M. von Köckritz-Blickwede, M. Dobbelstein, and D. Görlich, “Nanobodies to multiple spike variants and inhalation of nano-body-containing aerosols neutralize SARS-CoV-2 in cell culture and hamsters,” Antiviral Research, vol. 221, p. 105778, 2024. DOI: https://doi.org/10.1016/j.antiviral.2023.105778
J. Wang, B. Shi, H. Chen, M. Yu, P. Wang, Z. Qian, K. Hu, and J. Wang, “Engineered multivalent nanobodies efficiently neutralize SARS-CoV-2 omicron subvariants BA.1, BA.4/5, XBB.1 and BQ.1.1,” Vaccines (Basel), vol. 12, no. 4, 2024. DOI: https://doi.org/10.3390/vaccines12040417
G. Zhong, S. Xu, M. Cui, Q. Dong, X. Wang, Q. Xia, J. Gao, Y. Pei, Y. Qiao, and G. Pastel, “Rapid, high‐temperature, in situ microwave synthesis of bulk nanocatalysts,” Small, vol. 15, no. 47, p. 1904881, 2019. DOI: https://doi.org/10.1002/smll.201904881
V. Salema, and L. Á. Fernández, “Escherichia coli surface display for the selection of nanobodies,” Microbial biotechnology, vol. 10, no. 6, pp. 1468-1484, 2017. DOI: https://doi.org/10.1111/1751-7915.12819
Y.-J. Che, H.-W. Wu, L.-Y. Hung, H.-Y. Chang, K. Wang, and G.-B. Lee, "An integrated microfluidic system for screening of peptides specific to colon cancer cells and colon cancer stem cells using the phage display technology," vol. 5, no. 9. pp. 440-443, 2015. DOI: https://doi.org/10.1109/NEMS.2014.6908845
S. K. Burley, H. M. Berman, G. J. Kleywegt, J. L. Markley, H. Nakamura, and S. Velankar, “Protein Data Bank (PDB): the single global macromolecular structure archive,” Protein crystallography: methods and protocols, pp. 627-641, 2017. DOI: https://doi.org/10.1007/978-1-4939-7000-1_26
Y. Yan, H. Tao, J. He, and S.-Y. Huang, “The HDOCK server for integrated protein–protein docking,” Nature protocols, vol. 15, no. 5, pp. 1829-1852, 2020. DOI: https://doi.org/10.1038/s41596-020-0312-x
J. A. Maier, C. Martinez, K. Kasavajhala, L. Wickstrom, K. E. Hauser, and C. Simmerling, “ff14SB: improving the accuracy of protein side chain and backbone parameters from ff99SB,” Journal of chemical theory and computation, vol. 11, no. 8, pp. 3696-3713, 2015. DOI: https://doi.org/10.1021/acs.jctc.5b00255
P. Longsompurana, T. Rungrotmongkol, N. Plongthongkum, K. Wangkanont, P. Wolschann, and R. P. Poo-Arporn, “Computational design of novel nanobodies targeting the receptor binding domain of variants of concern of SARS-CoV-2,” Plos one, vol. 18, no. 10, p. e0293263, 2023. DOI: https://doi.org/10.1371/journal.pone.0293263
R. B. Best, X. Zhu, J. Shim, P. E. Lopes, J. Mittal, M. Feig, and A. D. MacKerell Jr, “Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ1 and χ2 dihedral angles,” Journal of chemical theory and computation, vol. 8, no. 9, pp. 3257-3273, 2012. DOI: https://doi.org/10.1021/ct300400x
H.-F. Tippmann, “Analysis for free: Comparing programs for sequence analysis,” Briefings in bioinformatics, vol. 5, no. 1, pp. 82-87, 2004. DOI: https://doi.org/10.1093/bib/5.1.82
R. A. Laskowski, J. Jabłońska, L. Pravda, R. S. Vařeková, and J. M. Thornton, “PDBsum: Structural summaries of PDB entries,” Protein science, vol. 27, no. 1, pp. 129-134, 2018. DOI: https://doi.org/10.1002/pro.3289
E. Gasteiger, C. Hoogland, A. Gattiker, S. e. Duvaud, M. R. Wilkins, R. D. Appel, and A. Bairoch, Protein identification and analysis tools on the ExPASy server: Springer, 2005. DOI: https://doi.org/10.1385/1-59259-890-0:571
M. Hebditch, M. A. Carballo-Amador, S. Charonis, R. Curtis, and J. Warwicker, “Protein–sol: A web tool for predicting protein solubility from sequence,” Bioinformatics, vol. 33, no. 19, pp. 3098-3100, 2017. DOI: https://doi.org/10.1093/bioinformatics/btx345
R. Deller, B. Carter, I. Zampetakis, F. Scarpa, and A. Perriman, “The effect of surface charge on the thermal stability and ice recrystallization inhibition activity of antifreeze protein III (AFP III),” Biochemical and biophysical research communications, vol. 495, no. 1, pp. 1055-1060, 2018. DOI: https://doi.org/10.1016/j.bbrc.2017.11.073
I. W. Davis, A. Leaver-Fay, V. B. Chen, J. N. Block, G. J. Kapral, X. Wang, L. W. Murray, W. B. Arendall, 3rd, J. Snoeyink, J. S. Richardson, and D. C. Richardson, “MolProbity: All-atom contacts and structure validation for proteins and nucleic acids,” Nucleic Acids Research, vol. 35, pp. W375-83, 2007. DOI: https://doi.org/10.1093/nar/gkm216
Z. Zhong, Y. Yang, X. Chen, Z. Han, J. Zhou, B. Li, and X. He, “Positive charge in the complementarity-determining regions of synthetic nanobody prevents aggregation,” Biochemical and Biophysical Research Communications, vol. 572, pp. 1-6, 2021. DOI: https://doi.org/10.1016/j.bbrc.2021.07.054
M. L. Fernández-Quintero, E. F. DeRose, S. A. Gabel, G. A. Mueller, and K. R. Liedl, “Nanobody paratope ensembles in solution characterized by MD simulations and NMR,” International Journal of Molecular Sciences, vol. 23, no. 10, p. 5419, 2022. DOI: https://doi.org/10.3390/ijms23105419
J. Lan, J. Ge, J. Yu, S. Shan, H. Zhou, S. Fan, Q. Zhang, X. Shi, Q. Wang, and L. Zhang, “Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor,” Nature, vol. 581, no. 7807, pp. 215-220, 2020. DOI: https://doi.org/10.1038/s41586-020-2180-5
Y. Wang, M. Liu, and J. Gao, “Enhanced receptor binding of SARS-CoV-2 through networks of hydrogen-bonding and hydrophobic interactions,” Proceedings of the National Academy of Sciences, vol. 117, no. 25, pp. 13967-13974, 2020. DOI: https://doi.org/10.1073/pnas.2008209117
T. R. Wagner, E. Ostertag, P. D. Kaiser, M. Gramlich, N. Ruetalo, D. Junker, J. Haering, B. Traenkle, M. Becker, and A. Dulovic, “NeutrobodyPlex—monitoring SARS‐CoV‐2 neutralizing immune responses using nanobodies,” EMBO reports, vol. 22, no. 5, p. e52325, 2021. DOI: https://doi.org/10.15252/embr.202052325
S. He, J. Wang, H. Chen, Z. Qian, K. Hu, B. Shi, and J. Wang, “A competitive panning method reveals an anti-SARS-CoV-2 nanobody specific for an RBD-ACE2 binding site,” Vaccines, vol. 11, no. 2, p. 371, 2023. DOI: https://doi.org/10.3390/vaccines11020371
X. Wu, L. Cheng, M. Fu, B. Huang, L. Zhu, S. Xu, H. Shi, D. Zhang, H. Yuan, and W. Nawaz, “A potent bispecific nanobody protects hACE2 mice against SARS-CoV-2 infection via intranasal administration,” Cell reports, vol. 37, no. 3, 2021. DOI: https://doi.org/10.1016/j.celrep.2021.109869
K. C. Entzminger, J. K. Fleming, P. D. Entzminger, L. Y. Espinosa, A. Samadi, Y. Hiramoto, S. C. Okumura, and T. Maruyama, “Rapid engineering of SARS-CoV-2 therapeutic antibodies to increase breadth of neutralization including BQ. 1.1, CA. 3.1, CH. 1.1, XBB. 1.16, and XBB. 1.5,” Antibody Therapeutics, vol. 6, no. 2, pp. 108-118, 2023. DOI: https://doi.org/10.1093/abt/tbad006
P. P. Pawar, and G. K. Bichile, “Molar extinction coefficients of some proteins,” Archives of Physics Research, vol. 2, no. 4, pp. 50-59, 2011.
B. M. Huyghues-Despointes, R. L. Thurlkill, M. D. Daily, D. Schell, J. M. Briggs, J. M. Antosiewicz, C. N. Pace, and J. M. Scholtz, “pK values of histidine residues in ribonuclease Sa: effect of salt and net charge,” Journal of molecular biology, vol. 325, no. 5, pp. 1093-1105, 2003. DOI: https://doi.org/10.1016/S0022-2836(02)01274-3
O. V. Sobolev, P. V. Afonine, N. W. Moriarty, M. L. Hekkelman, R. P. Joosten, A. Perrakis, and P. D. Adams, “A global ramachandran score identifies protein structures with unlikely stereochemistry,” Structure, vol. 28, no. 11, pp. 1249-1258.e2, 2020. DOI: https://doi.org/10.1016/j.str.2020.08.005
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












