Electrochemical study of Zn-Fe alloy coatings on mild steel for automotive applications
DOI:
https://doi.org/10.55713/jmmm.v35i2.2218Keywords:
Electrodeposition, Corrosion additive, Surface morphology, Micro-strainAbstract
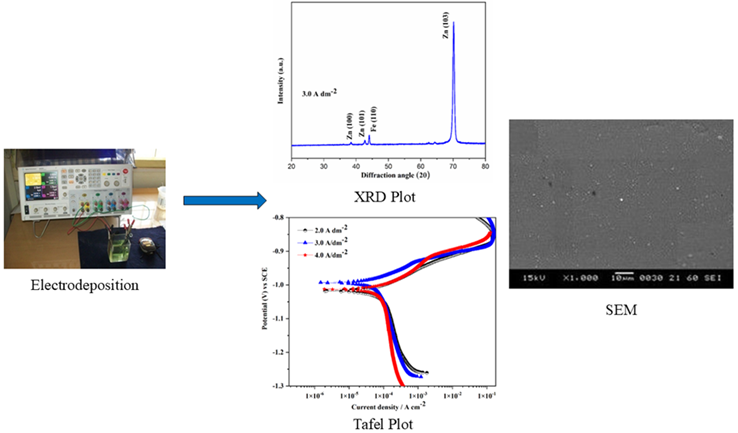
This study investigates the electrochemical behavior of Zn-Fe alloy deposited on mild steel (MS) substrates for automotive applications. The electrodeposition of a Zn-Fe alloy onto MS using an acid chloride bath, with 1,2,4-Triazole as an additive to enhance the uniformity of the deposit. The hull cell method was used to optimize the bath composition and operating conditions. The coatings were produced using electrodeposition at varying current densities, with 3 A∙dm‒2 identified as the optimal current density (CD) for achieving uniform coatings. The microstructural properties, including crystallite size and micro-strain, were analyzed using X-ray diffraction (XRD) and Williamson-Hall (W-H) analysis, revealing a homogenous distribution of crystallite size and strain. The impact of CD on coating features such as hardness, cathode current efficiency (CCE), thickness, and the weight % of metal contents was investigated. The corrosion resistance of the deposit was estimated using the potentiodynamic polarization and electrochemical impedance spectroscopy methods, and the results have been discussed. The structural and morphological properties of the deposit were investigated by Scanning electron microscopy (SEM). The roughness of the deposit was studied by Atomic force microscopy (AFM). The deposits containing Zn and Fe contents were confirmed by Energy-dispersive spectroscopy (EDS). The results suggest that Zn-Fe alloy coatings can provide superior protection for automotive components.
Downloads
References
J. Mackowiak, and N. R. Short, “Metallurgy of galvanized coatings,” International Metals Review, vol. 1, pp. 1-19, 1979. DOI: https://doi.org/10.1179/imtr.1979.24.1.1
T. V. Byk, T. V. Gaevskaya, and L. S. Tsybulskaya, “Effect of electrodeposition conditions on the composition, microstructure, and corrosion resistance of Zn-Ni alloy coatings,” Surface and Coatings Technology, vol. 202, pp. 5817-5823, 2008. DOI: https://doi.org/10.1016/j.surfcoat.2008.05.058
A. Tozar, and I. H. Karahan, “Structural and corrosion protection properties of electrochemically deposited nano-sized Zn-Ni alloy coatings,” Applied Surface Science, vol. 318, pp. 15-23, 2014.
A. P. Yadav, H. Katayama, K. Noda, H. Masuda, A. Nishikata, and T. Tsuru, “Effect of Fe-Zn alloy layer on the corrosion resistance of galvanized steel in chloride-containing environments,” Corrosion Science, vol. 49, pp. 3716-3731, 2007. DOI: https://doi.org/10.1016/j.corsci.2007.03.039
Z. Zhang, W. H. Leng, H. B. Shao, J. Q. Zhang, J. M. Wang, and C. N. Cao, “Study on the behavior of Zn–Fe alloy electro-plating,” Journal of Electroanalytical Chemistry, vol. 516, pp. 127-130, 2001. DOI: https://doi.org/10.1016/S0022-0728(01)00665-9
M. H. Gharahcheshmeh, and M. H. Sohi, “Study of the corrosion behavior of zinc and Zn-Co alloy electrodeposits obtained from the alkaline bath using direct current, Materials Chemistry and Physics, vol. 2, pp. 414-421, 2009. DOI: https://doi.org/10.1016/j.matchemphys.2009.06.009
M. M. Abou-Krishna, and A. M. Abushoffa, “Stripping voltammetric, conductance, and anodic linear polarization analysis on dissolution of electrodeposited zinc-cobalt alloy,” International Journal of Electrochemical Science, vol. 5, pp. 418-432, 2007. DOI: https://doi.org/10.1016/S1452-3981(23)17083-0
J. B. Bajat, S. Stankovic, and B. M. Jokic, “Electrochemical deposition, and corrosion stability of Zn–Co alloys,” Journal of Solid State Electrochemistry, vol. 13, pp. 755-762, 2009. DOI: https://doi.org/10.1007/s10008-008-0604-5
R. S.Bhat, and V. B. Shet, “Development and characterization of Zn-Ni, Zn-Co, and Zn-Ni-Co coatings,” Surface Engineering, vol. 36, pp. 429-437, 2020. DOI: https://doi.org/10.1080/02670844.2019.1680037
K. Venkatakrishna, A. C Hegde, and N. Eliaz, “Electrodeposition of bright Zn-Fe alloy on mild steel from acid chloride bath,” Indian Journal of Materials Science, vol. 5, pp. 289-298, 2009.
R. Ramanauskas, R. Juskenas, L. Kalinicenko, and F. Garfias-Mesias, “Microstructure, and corrosion resistance of electro-deposited zinc alloy coatings,” Journal of Solid State Electro-chemistry, vol. 8, pp. 416-421, 2004. DOI: https://doi.org/10.1007/s10008-003-0444-2
C. Q. Yang, Z. L. Long, and Y. C. Zhou, “Electrodeposition and physicochemical properties of Zn-Fe alloy coatings from sulfate solution,” Journal of Materials Science Letters, vol. 21, pp. 1677-1680, 2002. DOI: https://doi.org/10.1023/A:1020876726469
De Carvalho MF, E. P. Barbano, and I. A. Carlos, “Influence of disodium ethylene diamine tetraacetate on zinc electrodeposition process and the morphology, chemical composition, and structure of the electrodeposits,” Electrochimica Acta, vol. 109, pp. 798-808, 2013. DOI: https://doi.org/10.1016/j.electacta.2013.07.149
R. Bhat, U. K Bhat, and A. C. Hegde, “Optimization of deposition conditions for bright Zn-Fe coatings and its characterization,” Protection of Metals and Physical Chemistry of Surfaces, vol. 47, pp. 645-653, 2011. DOI: https://doi.org/10.1134/S2070205111050030
T. Boiadjieva-Scherzer, G. Avdeev, T. Vassilev, V. Chakarova, H. Kronberger, and M. Monev, “Influence of annealing temperature on ζ-CrZn13 formation in electrodeposited Zn–Cr coatings,” Surface Engineering, vol. 35, pp. 1055-1060, 2020. DOI: https://doi.org/10.1080/02670844.2019.1598023
C. Oulmas, S. Mameri, D. Boughrara, A. Kadri, J. Delhalle, Z. Mekhalif, and B. Benfedda, “Comparative study of Cu-Zn coatings electrodeposited from sulphate and chloride baths,” Heliyon, vol. 5, p. 02058, 2019. DOI: https://doi.org/10.1016/j.heliyon.2019.e02058
M. M. Abou-Krisha, A. M. Zaky, and A. A. Toghan, “Morphology, composition, and corrosion properties of electro-deposited Zn-Ni alloys from sulphate electrolytes,” Asian Journal of Biochemistry, vol. 1, pp. 84-97, 2006. DOI: https://doi.org/10.3923/ajb.2006.84.97
Y. T. Hsieh, R. W. Tsai, C. J. Su, and L. W. Sun, “Electro-deposition of Cu-Zn from chlorozincate ionic liquid: From hollow tubes to segmented nanowires, Journal of Physical Chemistry C, vol. 118, pp. 22347-22355, 2014. DOI: https://doi.org/10.1021/jp506833s
M. M. Abou-Krisha, F. H. Assaf, and A. A. Toghan, “Electro deposition of Zn-Ni alloys from sulfate bath,” Journal of Solid State Electrochemistry, vol. 11, pp. 244-252, 2007. DOI: https://doi.org/10.1007/s10008-006-0099-x
R. Solmaz, and B. D. Karahan, “Characterization, and corrosion studies of ternary Zn-Ni-Sn alloys,” International Journal of Minerals, Metallurgy and Materials, vol. 1, pp. 74-82, 2020. DOI: https://doi.org/10.1007/s12613-019-1888-4
R. S. Bhat, U. Bhat, and A. C. Hegde, “Corrosion behavior of electrodeposited Zn-Ni, Zn-Co, and Zn-Ni-Co alloys,” Analytical and Bioanalytical ElectroChemistry, vol. 3, pp. 302-315, 2011.
N. Eliaz, K. Venkatakrishna, and A. C. Hegde, “Electroplating and characterization of Zn-Ni, Zn-Co, and Zn-Ni-Co alloys,” Surface and Coatings Technology, vol. 205, pp. 1969-1978, 2010. DOI: https://doi.org/10.1016/j.surfcoat.2010.08.077
A. Toghan, M. M. Abou-krisha, F. H. Assaf, and F. El-Sheref, “Effect of deposition potential on the mechanism and corrosion behavior of Zn-Fe-Co thin coatings electrochemically deposited on a steel substrate,” International Journal of Electrochemical Science, vol. 16, p. 151044, 2021. DOI: https://doi.org/10.20964/2021.01.57
A. R. Marder, “The metallurgy of zinc-coated steel, Progress in Materials Science, vol. 45, pp. 191-271, 2000. DOI: https://doi.org/10.1016/S0079-6425(98)00006-1
K. R. Sriraman, S. Brahimi, J. A. Szpunar, J. H. Osborne, and S. Yue, “Characterization of corrosion resistance of electro-deposited Zn-Ni Zn and Cd coatings, Electrochimica Acta, vol. 105, pp. 314-323, 2013. DOI: https://doi.org/10.1016/j.electacta.2013.05.010
T. Y. Soror, and M. A. El-Ziady, “Effect of cetyl trimethyl ammonium bromide on the corrosion of carbon steel in acids,” Materials Chemistry and Physics, vol. 77, pp. 697-703, 2003. DOI: https://doi.org/10.1016/S0254-0584(02)00129-3
A. I . Vogel, “Quantitative inorganic analysis,” Longmans Green and Co., London,1951.
A. Brenner, “Electrodeposition of alloys,” Academic Press, New York, vol. 2, pp. 194, 1963. DOI: https://doi.org/10.1016/B978-1-4831-9807-1.50020-9
R. S. Bhat, S. M. Shetty, and N. V. Anil Kumar, “Electroplating of Zn-Ni alloy coating on mild steel and its electrochemical studies, Journal of Materials Engineering and Performance, vol. 30, pp. 8188-8195, 2021. DOI: https://doi.org/10.1007/s11665-021-06051-1
R. S. Bhat, K. B. Manjunatha, R. Prasanna Shankara, K. Venkatakrishna, and A. C. Hegde, “Electrochemical studies on the corrosion resistance of Zn-Ni-Co coating from acid chloride bath,” Applied Physics A, vol. 126, pp. 772, 2020. DOI: https://doi.org/10.1007/s00339-020-03958-9
A. Tozar, and I. H. Karahan, “Structural and corrosion protection properties of electrochemically deposited nano-sized Zn-Ni alloy coatings,” Applied Surface Science, vol. 318, pp. 15-23, 2014. DOI: https://doi.org/10.1016/j.apsusc.2013.12.020
J. B. Bajat, M. D. Maksimovic, V. B. Miskovic-Stankovic, and S. Zec, “Electrodeposition and characterization of Zn-Ni alloys as sublayers for epoxy coating deposition,” Journal of Applied Electrochemistry, vol. 31, pp. 355-361, 2001. DOI: https://doi.org/10.1023/A:1017580019551
A. Petrauskas, L. Grinceviciene, A. Cesuniene, and R. Juskenas, “Influence of Co2+ and Cu2+ on the phase composition of Zn-Ni alloys,” Electrochimica Acta, vol. 51, pp. 6135-6139, 2006. DOI: https://doi.org/10.1016/j.electacta.2006.01.064
J. B. Bajat, and V. B. Miskovic-Stankovic, “Protective properties of epoxy coatings electrodeposited on steel electrochemically modified by Zn-Ni alloys,” Progress in Organic Coatings, vol. 49, pp. 183-186, 2004. DOI: https://doi.org/10.1016/j.porgcoat.2003.09.019
M. M. Younan, “Surface microstructure and corrosion resistance of electrodeposited ternary Zn-Ni-Co alloy,” Journal of Applied Electrochemistry, vol. 30, pp. 55-60, 2000. DOI: https://doi.org/10.1023/A:1003840519591
A. T. Tabrizi, H. Aghajani, and F. F. Lelah, “Tribological characterization of hybrid chromium nitride thin layer synthesized on titanium,” Surface and Coatings Technology, vol. 15, p. 127317, 2021. DOI: https://doi.org/10.1016/j.surfcoat.2021.127317
M. Delaram, A. T. Tabrizi, and H. Aghajani, “Study the variation of surface topography & corrosion resistance of Cr-GO nano-composite coatings by addition of GO nanoparticles,” Surface Topography: Metrology and Properties, vol. 9, p. 015025, 2021. DOI: https://doi.org/10.1088/2051-672X/abe6f3
Y. Lin, and J. Selman, “Electrodeposition of corrosion‐resistant Ni‐Zn alloy: I. Cyclic voltammetric study,” Electrochemical Society, vol. 140, p. 1299, 1993. DOI: https://doi.org/10.1149/1.2220974
Z. Feng, Q. Li, J. Zhang, P. Yang, H. Song, and M. An, “Electro-deposition of nanocrystalline Zn–Ni coatings with single gamma phase from an alkaline bath,” Surface and Coatings Technology, vol. 47, p. 270, 2015. DOI: https://doi.org/10.1016/j.surfcoat.2015.03.020
Z. Feng, Q. Li, J. Zhang, P. Yang, and M. An, “Experimental and theoretical studies of DMH as a complexing agent for a cyanide-free gold electroplating electrolyte,” RSC Advances, vol. 5, p. 58199, 2015. DOI: https://doi.org/10.1039/C5RA13140E
Downloads
Published
How to Cite
License
Copyright (c) 2025 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












