Study of the effect of pH, conditioning and flotation time on the flotation efficiency of phosphate ores by a soybean oil collector
DOI:
https://doi.org/10.55713/jmmm.v32i1.1212Keywords:
Phosphate ore, Bio-flotation, Soybean oil collector, GangueAbstract
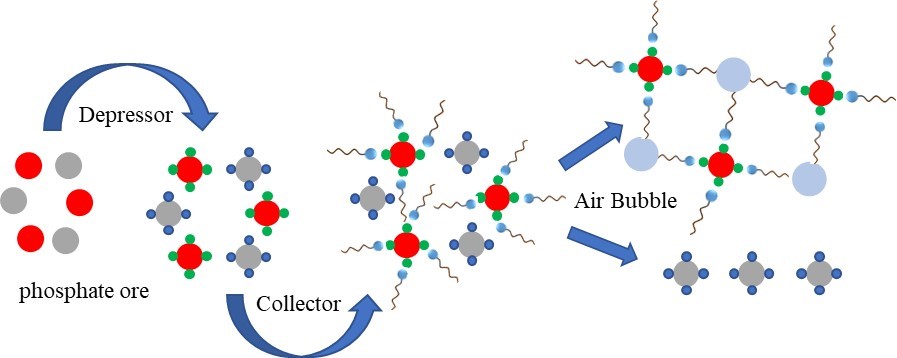
The mid-low grade of phosphate ore, rich by the silicate and carbonate gangue minerals needs a special treatment using the flotation process. Where the collectors play a pivotal role. This study was conducted to enrich an untreated phosphate ore (without washing, desliming, hydrocycling, etc.), with an eco-friendly and economic collector based on soybean oil. Different analytical techniques such as UV-Visible, ATR-FTIR spectrometric and XRD were used in order to analyse and characterize the flotation products. The effects of the parameters, pulp pH, conditioning time and flotation time on gangue removal efficiency by flotation, were investigated. Flotation tests were applied for granulometric class (90 mm to 125 µm). Consequently, a good quality phosphate concentrate was obtained in an acidic medium (pH = 4.05) with 1 g of soybean oil soap collector, 20 min flotation time, 10 min conditioning time, 0.17 L×min-1 air superficial velocity, 800 rpm at 25℃ and 6% solid content. The concentrate contained 29.0% phosphorus pentoxide (P2O5), with a recovery of 94.5% from a feed sample containing about 24.5% P2O5.
Downloads
References
Q. Cao, J. Cheng, S. Wen, C. Li, S. Bai, and D. Liu, “A mixed collector system for phosphate flotation,” Minerals Engineering, vol. 78, pp. 114-121, 2015.
M. Gharabaghi, M. Noaparast, and M. Irannajad, “Selective leaching kinetics of low-grade calcareous phosphate ore in acetic acid,” Hydrometallurgy, vol. 95, no. 3-4, pp. 341-345, 2009.
A. Z. M. Abouzeid, “Physical and thermal treatment of phosphate ores - An overview,” International Journal of Mineral Processing, vol. 85, no. 4, pp. 59-84, 2008.
S. Khoshjavan, and B. Rezai, “Beneficiation of refractory rock phosphate by calcination and flotation,” Mining, Metallurgy & Exploration, vol. 28, no. 4, pp. 187-192, 2011.
D. A. Elgillani, and A. Z. M. Abouzeid, “Flotation of carbonates from phosphate ores in acidic media,” International Journal of Mineral Processing, vol. 38, no. 3-4, pp. 235-256, 1993.
A. Z. M. Abouzeid, A. T. Negm, and D. A. Elgillani, “Upgrading of calcareous phosphate ores by flotation: Effect of ore characteristics,” International Journal of Mineral Processing, vol. 90, no. 1-4, pp. 81-89, 2009.
S. A. Abouel-enein, H. S. Gado, A. A. El-shennawy, A. M. Masoud, and A. A. Ahmed, “Phosphate Froth Flotation Using Sesame Oil as a Collector,” Journal of Basic and Environmental Sciences, vol. 7, pp. 106-123, 2020.
C. Owusu, K. Quast, and J. Addai-Mensah, “The use of canola oil as an environmentally friendly flotation collector in sulphide mineral processing,” Minerals Engineering, vol. 98, pp. 127-136, 2016.
E. P. Santos, A. J. B. Dutra, and J. F. Oliveira, “The effect of jojoba oil on the surface properties of calcite and apatite aiming at their selective flotation,” International Journal of Mineral Processing, vol. 143, pp. 34-38, 2015.
Y. Ruan, Z. Zhang, H. Luo, C. Xiao, F. Zhou, and R. Chi, “Ambient temperature flotation of sedimentary phosphate ore using cottonseed oil as a collector,” Minerals, vol. 7, no. 5, pp. 1-14, 2017.
P. Zhang, and R. Snow, “Studies of anionic reagents for phosphate beneficiation,” Mining, Metallurgy & Exploration, vol. 26, pp. 65-73, 2009.
T. E. Clemente, and E. B. Cahoon, “Soybean Oil: Genetic Approaches for Modification of Functionality and Total Content,” Plant Physiology, vol. 151, no. 3, pp. 1030-1040, 2009.
J. Kuligowski, G. Quintás, F. A. Esteve-Turrillas, S. Garrigues, and M. de la Guardia, “On-line gel permeation chromatography-attenuated total reflectance-Fourier transform infrared determination of lecithin and soybean oil in dietary supplements,” Journal of Chromatography A, vol. 1185, no. 1, pp. 71-77, 2008.
M. J. Lerma-García, G. Ramis-Ramos, J. M. Herrero-Martínez, and E. F. Simó-Alfonso, “Authentication of extra virgin olive oils by Fourier-transform infrared spectroscopy,” Food Chemistry, vol. 118, no. 1, pp. 78-83, 2010.
J. V. Kadamne, V. P. Jain, M. Saleh, and A. Proctor, “Measurement of Conjugated Linoleic Acid (CLA) in CLA-Rich Soy Oil by Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR),” Journal of Agricultural and Food Chemistry, vol. 57, pp. 10483-10488, 2009.
T. Furuzono, D. Walsh, K. Sato, K. Sonoda, and J. Tanaka, “Effect of reaction temperature on the morphology and size of hydroxyapatite nanoparticles in an emulsion system,” Journal of Materials Science Letters, vol. 20, no. 2, pp. 111-114, 2001.
M. Dakkach, A. Atlamsani, and S. Sebti, “Natural phosphate modified by vanadium: A new catalyst for oxidation of cycloalkanones and α-ketols with oxygen molecular,” Comptes Rendus Chimie, vol. 15, no. 6, pp. 482-492, 2012.
M. Sadiq, M. Abdennouri, N. Barka, M. Baalala, C. Lamonier, and M. Bensitel, “Influence of the Crystal Phase of Magnesium Phosphates Catalysts on the Skeletal Isomerization of 3,3-dimethylbut-1-ene,” Canadian Chemical Transactions, vol. 3, no. 2, pp. 225-233, 2015.
C. Drouet, “Apatite formation: Why it may not work as planned, and how to conclusively identify apatite compounds,” BioMed Research International, vol. 2013, no. 4, pp. 1-12, 2013.
E. M. Zahrani, and M. H. Fathi, “The effect of high-energy ball milling parameters on the preparation and characterization of fluorapatite nanocrystalline powder,” Ceramics International, vol. 35, pp. 2311-2323, 2009.
I. Nikcevic, V. Jokanovic, M. Mitric, Z. Nedic, D. Makovec, and D. Uskokovic, “Mechanochemical synthesis of nano-structured fluorapatite/fluorhydroxyapatite and carbonated fluorapatite/fluorhydroxyapatite,” Journal of Solid State Chemistry, vol. 177, pp. 2565-2574, 2004.
M. E. Fleet, “Infrared spectra of carbonate apatites: ν2-Region bands,” Biomaterials, vol. 30, no. 8, pp. 1473-1481, 2009.
M. Wang, R. Qian, M. Bao, C. Gu, and P. Zhu, “Raman, FT-IR and XRD study of bovine bone mineral and carbonated apatites with different carbonate levels,” Materials Letters, vol. 210, pp. 203-206, 2018.
R. Yous, F. Mohellebi, H. Cherifi, and A. Amrane, “Competitive biosorption of heavy metals from aqueous solutions onto Streptomyces rimosus,” Korean Journal of Chemical Engineering, vol. 35, pp. 890-899, 2018.
R. D. Aines, and G. R. Rossman, “Water in Minerals? A Peak in the Infrared,” Journal of Geophysical Research: Solid Earth, vol. 89, no. B6, pp. 4059-4071, 1984.
K. Boughzala, and K. Bouzouita, “Synthesis and characterization of strontium–calcium–lanthanum apatites Sr7-xCaxLa3(PO4)3(SiO4)3F2 0≤ x ≤ 2,” Comptes Rendus Chimie, vol. 18, no. 8, pp. 858-866, 2015.
C. E. Jordan, and D. R. Spears, “Evaluation of a turbulent flow model for fine-bubble and fine-particle flotation,” Mining, Metallurgy & Exploration, vol. 7, pp. 65-73, 1990.
D. Henwood, “The effect of conditioning on froth flotation,” Dissertations, cape town University, July 1994.
S. R. Rao, “Surface Chemistry of Froth Flotation,” Volume 1: Fundamentals New York, 2004.
L. Zhang, “Enhanced phosphate flotation using novel depressants,” Theses and Dissertations-Mining Engineering 10, Kentucky University, 2013.
Y. Ruan, Z. Zhang, H. Luo, C. Xiao, F. Zhou, and R. Chi, “Effects of metal ions on the flotation of apatite, dolomite and quartz,” Minerals, vol. 8, no. 4, pp. 1-12, 2018.
M. Prasad, A. K. Majumder, and T. C. Rao, “Reverse flotation of sedimentary calcareous/dolomitic rock phosphate ore - an overview,” Mining, Metallurgy & Exploration, vol. 17, no. 1, pp. 49-55, 2000.
X. Shao, C. L. Jiang, and B. K. Parekh, “Enhanced flotation separation of phosphate and dolomite using a new amphoteric collector,” Mining, Metallurgy & Exploration, vol. 15, pp. 11-14, 1998.
Z. E. Öztin, “Parametric studies on cell flotation of mazidaği phosphate rock,” PhD Thesis, Middle East Technical University, September 2003.
I. Birken, M. Bertucci, J. Chappelin, and E. Jorda, “Quantification of impurities, including carbonates speciation for phosphates beneficiation by flotation,” Procedia Engineering, vol. 138, pp. 72-84, 2016.
H. El Feki, C. Rey, and M. Vignoles, “Carbonate Ions in Apatites: Infrared Investigations in the 4 CO3 Domain,” Calcified Tissue International, vol. 49, pp. 269-274, 1991.
C. Rey, V. Renugopalakrishnan, M Shimizu, B. Collins, and M. Glimcher, “A resolution-enhanced Fourier transform Infrared spectroscopy of the environment of the CO32- ion in the mineral phase of enamel during its formation and maturation,” Calcified Tissue International, vol. 49, pp. 259-268, 1991.
A. Kenzour, H. Belhouchet, M. Kolli, S. Djouallah, and D. Kherifi, S. Ramesh, “Sintering behavior of anorthite-based composite ceramics produced from natural phosphate and kaolin,” Ceramics International, vol. 45, pp. 20258-20265, 2019.
W. Gallala, F. Herchi, I. Ben Ali, L. Abbassi, M. E. Gaied, and M. Montacer, “Beneficiation of Phosphate Solid Coarse Waste from Redayef (Gafsa Mining Basin) by Grinding and Flotation Techniques,” Procedia Engineering, vol. 138, pp. 85-94, 2016.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












