Investigating the effect of temperature and NaCl concentration on corrosion behavior using commercial food cans
DOI:
https://doi.org/10.55713/jmmm.v33i1.1595Keywords:
Corrosion, Food Cans, Tin-plate, Chloride acidAbstract
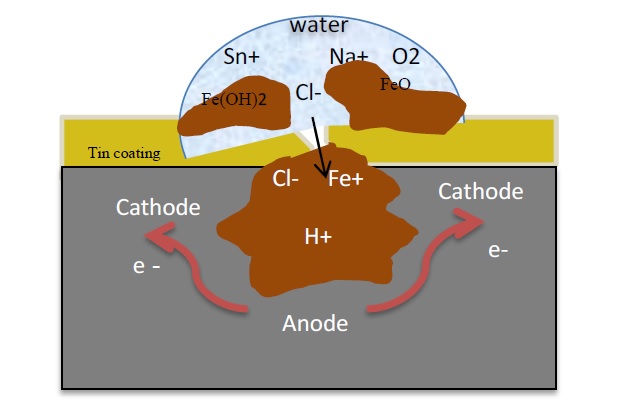
In the present work, commercial tin-plate used as storage cans for three different products (salmon fish, green peas, and corn) are used to investigate the corrosion to storage temperature and salt concentration. The corrosion process was electrochemically monitored using the potentiodynamic polarization method and the structure and concentrations of the corrosion products were investigated using X-ray diffraction (XRD). To characterize the resulting sample morphology, emission scanning electron microscopy (FESEM) was used. The results showed that the corrosion resistance of the tin-coating, was important constant and by changing the NaCl concentration and operation temperature, the corrosion resistance was lower. It is believed that the steel substrate dissolution that is underneath the tin coating is the main driving force for the investigated corrosion process. The experimental results show that the operating temperature has a higher impact on the corrosion rate because it promotes a heavy tendency on the rate of diffusion of molecules or ions in a solution. Green peas tin-plates are corroded more than corn, and salmon fish tin-plates when the temperature was increased from 25℃ to 50℃ and showed higher negative corrosion potential and higher corrosion current density in sodium chloride solutions.
Downloads
References
Y. Xia, S. Zhang, L. Tong, S. Liu, G. Lin, Z. Bai, Y. He, and Z. Liu, “Introducing cyano-functionalized multiwalled carbon nanotubes to improve corrosion resistance and mechanical performance of poly(arylene ether nitrile) coating,” Surface and Coatings Technology, vol. 432, p. 128058, 2022.
Y. Chen, W. Bai, J. Chen, X. Chen, J. Zhao, F. Wei, R-K. Jian, X. Zheng, and Y. Xu,“In-situ intercalation of montmorillonite/ urushiol titanium polymer nanocomposite for anti-corrosion and anti-aging of epoxy coatings,” Progress in Organic Coatings, vol. 165, p. 106738, 2022.
F. Su, X. Du, T. Shen, A. Qin, and W. Li, “Aggregation-induced emission luminogens sensors: Sensitive fluorescence ‘Turn-On’ response for pH and visually chemosensoring on early detection of metal corrosion,” Progress in Organic Coatings, vol. 153, p. 106122, 2021.
H. Zheng, W. Liu, S. He, R. Wang, J. Zhu, X. Guo, N. Liu, R. Guo, and Z. Mo, “A superhydrophobic polyphenylene sulfide composite coating with anti-corrosion and self-cleaning properties for metal protection,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 648, p. 129152, 2022.
C.-M. Deng, Y. Zhu, S. Sun, J. Wei, and D.-H. Xia, “Analysis of failure causes of epoxy-phenolic coated tinplate after boiling sterilization,” Engineering Failure Analysis, vol. 135, p. 106129, 2022.
L. Guo, H. Wang, X. Li, G. Fei, Y. Yuan, and Y. Li, “A synergistic system of polyaniline@ graphene-alkyd resin via a Gemini surfactant for enhanced anti-corrosion properties,” Progress in Organic Coatings, vol. 170, p. 106944, 2022.
A. Marotta, N. Faggio, V. Ambrogi, A. Mija, G. Gentile, and P. Cerruti, “Biobased furan-based epoxy/TiO2 nanocomposites for the preparation of coatings with improved chemical resistance,” Chemical Engineering Journal, vol. 406, p. 127107, 2021
W. Liu, Z. Mo, C. Shuai, S. He, R. Yue, X. Guo, Y. Chen, H. Zheng, J. Zhu, R. Guo, and N. Liu, “Fabrication of TiO2/CeO2/ PPS corrosion protective hydrophobic coating by air spraying,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 647, p. 129056, 2022.
S. Liu, H. Xe, Q. Cao, Y. Ning, Y. Song, C. Zhand, and B. Liu, “Preparation of a novel IPDI/PUF@CeO2 bi-functional microcapsules and its improvement for the self-healing and anti-corrosion performance in epoxy coatings,” Progress in Organic Coatings, vol. 169, p. 106897, 2022.
E. Zumelzu, and C. Cabezas, “Observations on the influence of microstructure on electrolytic tinplate corrosion,” Materials Characterization, vol. 34, no. 2, pp. 143-148, 1995.
G. G. D. Nascimento, J. L. C. D. Santos, I. C. P. Margarit, and O. R. Mattos, “Lacquered tinplate: Corrosion resistance in the function of lacquering conditions,” Electrochimica Acta, vol. 41, no. 7-8, pp. 1099-1102, 1996.
D. H. Xia, J. H. Wang, S. Z. Song, B. Zhong, and Z. W. Han, “The corrosion behavior of lacquered tinplate in functional beverage,” Advanced Materials Research, vol. 233-235, pp. 1747-1751, 2011.
J. E. Charbonneau, “Recent case histories of food product ‐metal container interactions using scanning electron microscopy‐x‐ray microanalysis,” Scanning, vol. 19, no. 7, pp. 512-518, 1997.
X. Huang, N. Li, L. Cao, and J. Zheng, “Electrodeposited lanthanum film as chromate replacement for tinplate,” Materials Letters, vol. 62, no. 3, pp. 466-469, 2008.
B. X. Huang, P. Tornatore, and Y.-S. Li, “IR and Raman spectroelectrochemical studies of corrosion films on tin,” Electrochimica Acta, vol. 46, no. 5, pp. 671-679, 2001.
A. N. Grassino, Z. Grabarić, A. Pezzani, G. Squitieri, and K. Berković, “Corrosion inhibition with different protective layers in tinplate cans for food preservation,” Journal of the Science of Food and Agriculture, vol. 90, no. 14, pp. 2419-2426, 2010.
R. Catalá, M. Alonso, R. Gavaram E. Almeida, J. Bastidas, J. M. Puente, and N. de Cristaforo,“Titanium-passivated tinplate for canning foods,” Food Science and Technology International, vol. 11, no. 3, pp. 223-227, 2005.
C. A. Gervasi, P. A. Palacios, M. v Fiori Bimbi, and P. E. Alvarez, “Electrochemical studies on the anodic behavior of tin in citrate buffer solutions,” Journal of Electroanalytical Chemistry, vol. 639, no. 1-2, pp. 141-146, 2010.
O. Hazzazi, “On the pitting corrosion behaviour of pure Al by halide ions in neutral sulphate solutions and the effect of some inorganic inhibitors,” Journal of King Abdulaziz University-Science, vol. 19, no. 1, pp. 47-65, 2007.
G. W. Patrick, “Internal corrosion of tinplate food containers,” Anti-Corrosion Methods and Materials, vol. 23, no. 6, pp. 9-11, 1976.
A. S. Tselesh, “Anodic behaviour of tin in citrate solutions: The IR and XPS study on the composition of the passive layer,” Thin Solid Films, vol. 516, no. 18, pp. 6253-6260, 2008.
S. A. M. Refaey, and G. Schwitzgebel, “Electrochemical impedance spectroscopic investigation of dissolution, passivation and pitting corrosion of tin in Na2CO3 solution and the effect of Cl− and I− ions,” Applied Surface Science, vol. 135, no. 1-4, pp. 243-253, 1998.
M. Jafarian, F. Gobal, I. Danaee, R. Biabani, and M. G. Mahjani, “Electrochemical studies of the pitting corrosion of tin in citric acid solution containing Cl−,” Electrochimica Acta, vol. 53, no. 13, pp. 4528-4536, 2008.
G. Urbano, V. E. Reyes, M. A. Veloz, and I. González, “Pyrite−arsenopyrite galvanic interaction and electrochemical reactivity,” The Journal of Physical Chemistry C, vol. 112, no. 28, pp. 10453-10461, 2008.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












