Optimized conditions for cobalt diffusion in Sri Lankan colorless topaz and coloration mechanism elucidation through spectro-chemical investigation
DOI:
https://doi.org/10.55713/jmmm.v33i1.1596Keywords:
Co diffusion, Blue Topaz, Heat Treatment, Coloration of blue topazAbstract
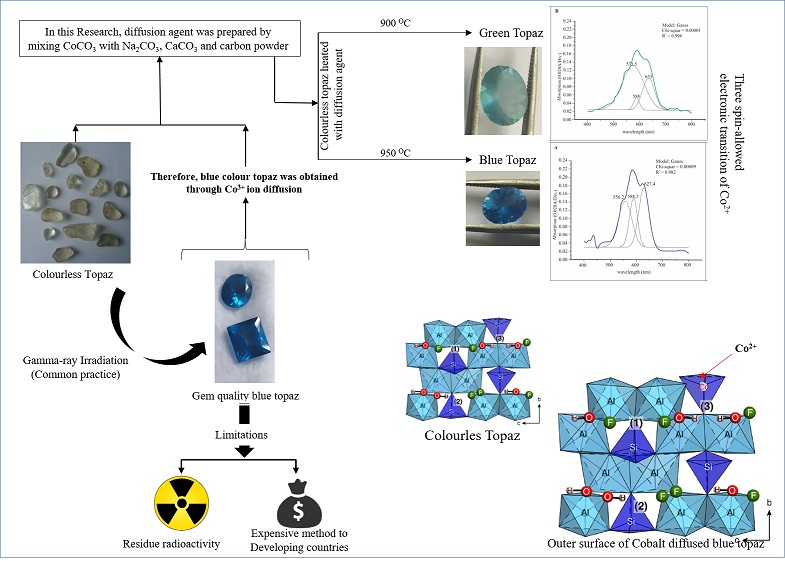
Most of natural topaz is colorless; thus, methods of color enhancement are widely used for coloring this mineral. Currently, blue color is obtained by cobalt diffusion due to drawbacks in existing coloration methods. In this study, optimum conditions suitable for Cobalt diffusion in Sri Lankan colorless topaz were investigated and coloration mechanism was elucidated. The diffusion agent was prepared by mixing CoCO3 with Na2CO3, CaCO3 and carbon powder and diffusion was carried-out by varying the temperature and soaking time. Chemical analysis, UV-Vis absorption spectrum, infrared absorption spectra, and Raman peaks of diffused and non-diffused topaz were tested. The results clearly indicated that the optimum condition for Co diffusion in Sri Lankan topaz is 950℃ for 11 h. The EPMA analysis showed that the Co concentration in the diffused sample varied from 0.001 wt% to 0.027 wt% while colorless topaz showed <0.001 wt%. The UV-Vis spectrum of Co diffused blue topaz gave three absorption peaks at 556, 588, and 627 nm corresponding to three spin-allowed electronic transitions of Co2+ ion in teterahedaral coordination. In case of Co diffused topaz, one additional new broader IR absorption peak was noticed around 6640 cm-1 presumably arising by optical transitions of 4A2 → 4T1 in Co2+ (4F). Our results lead to the conclusion that, blue color of the Co diffused topaz is arising by spin-allowed electronic transitions of Co2+ ions in tetrahedral site of topaz matrix through substitution of Si4+ ions.
Downloads
References
M. V. B. Pinheiro, C. Fantini, K. Krambrock, A. I. C. Persiano, M. S. S. Dantas, and M. A. Pimenta, “OH/F substitution in topaz studied by Raman spectroscopy,” Physical Review B - Condensed Matter and Materials Physics, vol. 65, pp. 1-6, 2002.
M. Gaft, R. Reisfeld, and G. Panczer, Modern Luminescence Spectroscopy of Minerals and Materials, Second Edi. Springer International Publishing Switzerland, 2015.
D. N. Da Silva, K. J. Guedes, M. V. B. Pinheiro, S. Schweizer, J. M. Spaeth, and K. Krambrock, “The O- (Al2) centre in topaz and its relation to the blue colour,” Physica Status Solidi C: Conferences, vol. 2, pp. 397-400, 2005.
K. Boonsook, W. Kaewwiset, P. Limsuwan, and K. Naemchanthara, “Gamma ray evaluation of fast neutron irradiated on topaz from Sri Lanka by HPGe gamma ray spectrometry,” Journal of Physics: Conference Series, vol. 901, 2017.
H. Gabasch, F. Klauser, E. Bertel, and T. Rauch, “Coloring of topaz by coating and diffusion processes: An X-Ray photoemission study of what happens beneath the surface,” Gems & Gemology, vol. 44, pp. 148-154, 2008.
R. Pollak, “Method for enhancing the color of minerals useful as gemstones,” 1997.
P. P. Rout, R. K. Sahoo, S. K. Singh, and B. K. Mishra, “Spectroscopic investigation and colour change of natural topaz exposed to PbO and CrO3 vapour,” Vibrational Spectroscopy, vol. 88, pp. 1-8, 2017.
K. Nssau, and B. E. Pnnscorr, “Blue and brown topaz produced by gamma lrradiation,” American M ine ralogist, vol. 60, pp. 705-709, 1975.
A. R. P. L. Albuquerque, S. Isotani, and S. P. Morato, “Irradiation and heating effects in topaz crystals from minas cerais, Brazil,” Radiation Effects, vol. 106, pp. 143-150, 1988,
K. Nassau, “Altering the color of topaz,” Gems & Gemology, vol. 21, pp. 26-34, 1985.
A. Maneewong, K. Pangza, T. Charoennam, N. Thamrongsiripak, and N. Jangsawang, “The impact of electron beam irradiation in topaz quality enhancement,” Journal of Physics: Conference Series, vol. 1285, pp. 1-7, 2019.
N. M. A. Mohamed, and M. A. Gaheen, “Design of fast neutron channels for topaz irradiation,” Nuclear Engineering and Design, vol. 310, pp. 429-437, 2016.
C. E. Ashbaugh, and james E. Shigley, “Reactor-irradiated green topaz,” Gems & Gemology, vol. 29, pp. 116-121, 1993.
E. H. Christiansen, D. M. Burt, M. F. Sheridan, and R. T. Wilson, “The petrogenesis of topaz rhyolites from the western United States,” Contributions to Mineralogy and Petrology, vol. 83, pp. 16-30, 1983.
S. Illangasinghe, S. Wickramarathna, S. Diyabalanage, L. Herath, P. Francis, and C. Jaliya, “Gem Trader’s perception on treatment of low gem quality minerals, ratnapura, sri lanka,” in International Research Conference of UWU-2019, 2019, p. 474. Accessed: Apr. 07, 2020. [Online]. Available: http://www.erepo.lib.uwu.ac.lk/ handle/123456789/672
V. Skvortsova, N. Mironova- Ulmane, L. Trinkler, and G. Chikvaidze, “Optical properties of natural topaz,” IOP Conference Series: Materials Science and Engineering, vol. 49, 2013.
A. C. S. Sabioni, G. M. da Costa, J. M. Dereppe, C. Moreaux, and C. M. Ferreira, “Behaviour of Brazilian imperial topaz at high temperature,” The Journal of Gemmology, vol. 28, pp. 283-290, 2003.
M. S. Hampar and J. Zussman, “The thermal breakdown of Topaz,” TMPM Tschermaks Mineralogische und Petrographische Mitteilungen, vol. 33, pp. 235-252, 1984.
R. A. Day, E. R. Vance, D. J. Cassidy, and J. S. Hartman, “The topaz to mullite transformation on heating,” Journal of Materials Research, vol. 10, pp. 2963-2969, 1995.
R. W. Hughes, and T. Underwood, “Surface enhanced topaz,” Journal of the Accredited Gemologist Association, vol. Winter, pp. 1-8, 1999.
F. Colombo, R. Lira, and E. V Pannunzio Miner, “Mineralogical characterization of topaz from miarolitic pegmatites and w-bearing greisen in the a-type el portezuelo granite, papachacra (Catamarca Province),” Revista de la Asociación Geológica Argentina, vol. 64, pp. 193199, 2009.
T. Gauzzi, L. M. Graça, L. Lagoeiro, I. de Castro Mendes, and G. N. Queiroga, “The fingerprint of imperial topaz from Ouro Preto region (Minas Gerais state, Brazil) based on cathodoluminescence properties and composition,” Mineralogical Magazine, vol. 82, pp. 943-960, 2018.
P. P. Rout, R. K. Sahoo, S. K. Singh, and B. K. Mishra, “Spectroscopic investigation and colour change of natural topaz exposed to PbO and CrO3 vapour,” Vibrational Spectroscopy, vol. 88, pp. 1-8, 2017.
G. D. Gatta, F. Nestola, G. D. Bromiley, and S. Loose, “New insight into crystal chemistry of topaz: A multi-methodological study,” American Mineralogist, vol. 91, p. 1839ñ1846, 2006.
A. Agangi, A. Gucsik, H. Nishido, K. Ninagawa, and V. S. Kamenetsky, “Relation between cathodoluminescence and trace-element distribution of magmatic topaz from the Ary-Bulak massif, Russia,” Mineralogical Magazine, vol. 80, pp. 881-899, 2016.
G. Lehmann, “Interstitial incorporation of di- and trivalent cobalt in quartz,” Journal of Physics and Chemistry of Solids, vol. 30, pp. 395-399, 1969.
V. D’Ippolito, G. B. Andreozzi, U. Hålenius, H. Skogby, K. Hametner, and D. Günther, “Color mechanisms in spinel: cobalt and iron interplay for the blue color,” Physics and Chemistry of Minerals, vol. 42, pp. 431-439, 2015.
L. C. B. d. M. Pinto, A. Righi, F. S. Lameiras, F. G. S. da Araujo, and K. Krambrock, “Origin of the color in cobalt-doped quartz,” Physics and Chemistry of Minerals, vol. 38, pp. 623-629, 2011.
M. N. Taran, M. Koch-Müller, and A. Feenstra, “Optical spectroscopic study of tetrahedrally coordinated Co2+ in natural spinel and staurolite at different temperatures and pressures,” American Mineralogist, vol. 94, pp. 1647-1652, 2009.
P. J. Dereń, W. Strȩk, U. Oetliker, and H. U. Güdel, “Spectroscopic properties of Co2+ ions in MgAl2O4 spinels,” physica status solidi (b), vol. 182, pp. 241-251, 1994.
B. Chauviré, B. Rondeau, E. Fritsch, P. Ressigeac, and J.-L. Devidal, “Blue spinel from the luc yen district of Vietnam | gems & Gemology,” Gems & Gemology, vol. 51, 2015.
J. Popović, E. Tkalčec, B. Gržeta, and B. Rakvin, “Inverse spinel structure of Co-doped gahnite,” American Mineralogist, vol. 94, pp. 771-776, 2009.
R. G. Burns, Mineralogical applications of crystal field theory. Second edition. Cambridge University Press; Cambridge Topics in Mineral Physics and Chemistry, 5, 1993.
P. J. Alonso, and R. Alcalá, “On the optical absorption spectrum of Co2+ in CaF2,” Physica Status Solidi (b), vol. 81, pp. 333-339, 1977.
D. N. Souza, J. F. de Lima, M. E. G. Valerio, C. Fantini, M. A. Pimenta, R. L. Moreira, and L. V. E. Caldas,“Influence of thermal treatment on the Raman, infrared and TL responses of natural topaz,” Nuclear Instruments and Methods in Physics Research, Section B, vol. 191, pp. 230-235, 2002.
C. P. Smith, “A contribution to understanding the infrared spectra of rubies from Mong Hsu, Myanmar,” The Journal of Gemmology, vol. 24, pp. 321-335, 1995.
S. V Churakov, and A. B. Wunder, “Ab-initio calculations of the proton location in topaz-OH, Al2SiO4 (OH)2,” Physics and Chemistry of Minerals, vol. 31, pp. 131-141, 2004.
C. Paikaew, J. Inthanont, A. Punyanut, E. Hoonnivathana, P. Limsuwan, and K. Naemchanthara, “Effects of electron beam on structure and physical properties of natural colorless topaz,” Advanced Materials Research, vol. 1125, pp. 60-63, 2015.
K. Komatsu, H. Kagi, T. Okada, T. Kuribayashi, J. B. Parise, and Y. Kudoh, “Pressure dependence of the OH-stretching mode in F-rich natural topaz and topaz-OH,” American Mineralogist, vol. 90, pp. 266-270, 2005.
C. A. Londos, A. Vassilikou‐Dova, G. Georgiou, and L. Fytros, “Infrared studies of natural topaz,” Physica Status Solidi (a), vol. 133, pp. 473-479, 1992.
P. S. R. Prasad, and T. N. Gowd, “FTIR spectroscopic study of hydroxyl ions in natural topaz,” Journal of the Geological Society of India, vol. 61, pp. 202-208, 2003.
J. M. Beny, and B. Piriou, “Vibrational spectra of single-crystal topaz,” Physics and Chemistry of Minerals, vol. 15, pp. 148-159, 1987.
R. D. Aines, and G. R. Rossman, “Relationships between radiation damage and trace water in zircon, quartz, and topaz,” American Mineralogist, vol. 71, pp. 1186-1193, 1986.
A. Watenphul, and B. Wunder, “Temperature dependence of the OH-stretching frequencies in topaz-OH,” Physics and Chemistry of Minerals, vol. 37, pp. 65-72, 2010.
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












