Surface modification of activated carbon from sago waste
DOI:
https://doi.org/10.55713/jmmm.v33i1.1616Keywords:
activated carbon, oxidative modification, porosity properties, sago wasteAbstract
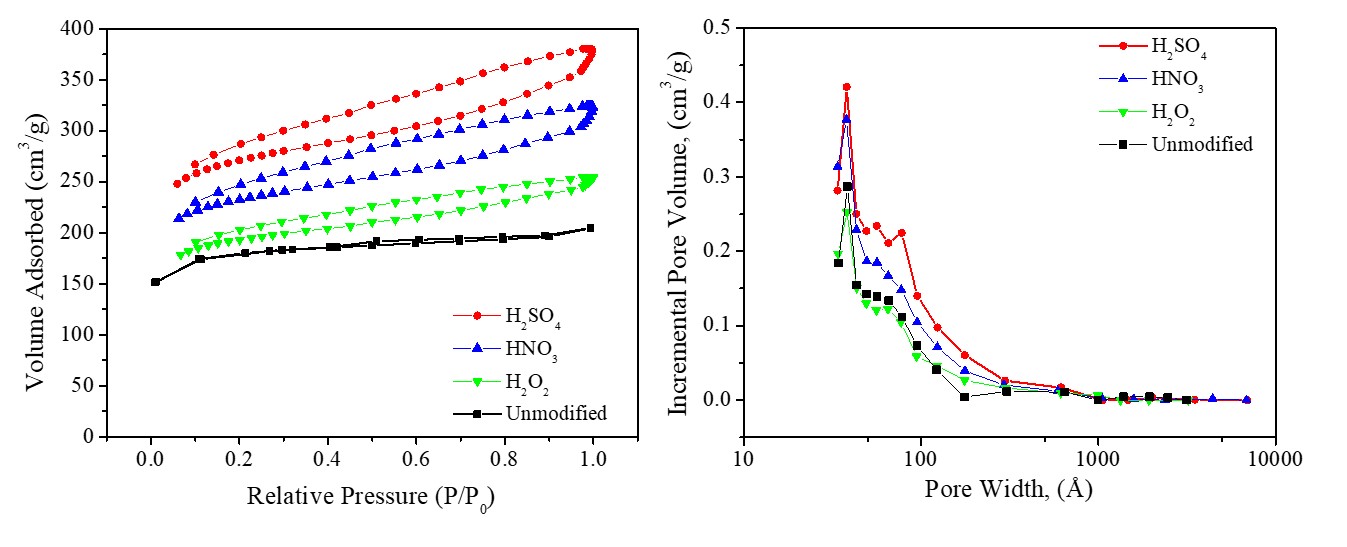
In this paper, we analyzed the effect of surface modification on the surface properties of the active carbon from sago waste using varied oxidizers, namely H2O2, HNO3, and H2SO4. The ordinary active carbon has an initial surface area of 546.6 m2∙g-1, with a phenol and carboxylic functional group. The porosity, functional groups and morphological surface, together with the chemical composition of activated carbon were examined using a nitrogen adsorption-desorption through the Brunauer Emmett Teller (BET) method and the calculation of Barret-Joyner-Hall (BJH), a Fourier-transformed infrared spectroscopy, and a scanning electron microscopy with energy dispersive spectroscopy. The results found that the modified activated carbon significantly increased surface area and total pore volume. Activated carbon modified using H2SO4 oxidizers has the highest surface area value of 853.6 m2∙g-1 and a total pore volume value of 0.585 cm3∙g-1. In addition, the surface modification has changed carbon's porosity from micropore to mesopore, altered the surface functional group from phenol to ether. The surface modification has improved its adsorption capacity and potentially further its application. In conclusion, modifying the surface could make the properties closer to the standards for commercial activated carbon.
Downloads
References
P. González-García, "Activated carbon from lignocellulosic precursors: A review of the synthesis methods, characterization techniques, and applications," Renewable and Sustainable Energy Reviews, vol. 82, pp. 1393-1414, 2018.
L. S. Blankenship, N. Balahmar, and R. Mokaya, "Oxygen-rich microporous carbons with exceptional hydrogen storage capacity," Nature Communications, vol. 8, pp. 1-12, 2017.
I. Demiral, C. Samdan, and H. Demiral, "Enrichment of the surface functional groups of activated carbon by modification method," Surfaces and Interfaces, vol. 22, p. 100873, 2021.
O. Togibasa, Y. O. Ansanay, K. Dahlan, and M. Erari, "Identification of surface functional group on activated carbon from waste sago," Journal of Physics: Theories and Applications, vol. 5, pp. 1-8, 2021.
M. Mariana, H. P. S Abdul Khalil, E. M. Mistar, E. B. Yahya, T. Alfatah, M. Danish, and M. Amayreh, "Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption," Journal of Water Process Engineering, vol. 43, p. 102221, 2021.
N. F. Attia, S. M. Shaltout, I. A. Salem, A. B. Zaki, M. H. El-Sadek, and M. A. Salem, "Sustainable and smart hybrid nano-porous adsorbent derived biomass as efficient adsorbent for cleaning of wastewater from Alizarin Red dye," Biomass Conversion and Biorefinery, pp. 1-16, 2022.
M. Jung, J. Park, K. Lee, F. N. F. Attia, and H. Oh, "Effective synthesis route of renewable nanoporous carbon adsorbent for high energy gas storage and CO2/N2 selectivity," Renewable Energy, vol. 161, pp. 30-42, 2020.
S. I. El-Hout, S. Y. Attia, S. G. Mohamed, and S. M. Abdelbasir, "From waste to value-added products: Evaluation of activated carbon generated from leather waste for supercapacitor applications," Journal of Environmental Management, vol. 304, p. 114222, 2022.
Y. K. Allo, Sudarmono, and O. Togibasa, "Synthesis and characterization of activated carbon from sago waste (Metroxylon sagu) with ZnCl2 activation and HNO3 modification," Journal of the Indonesian Chemical Society, vol. 2, pp. 48-53, 2019.
O. Togibasa, M. Mumfaijah, Y. K. Allo, K. Dahlan, Y. O. Ansanay, "The effect of chemical activating agent on the properties of activated carbon from sago waste," Applied Sciences, vol. 11, pp. 11640, 2021.
J. C. Lai, W. A. W. A. Rahman, W. Y. Toh, "Characterization of sago pith waste and its composites," Industrial Crops and Products, vol. 45, pp. 319-326, 2013.
P. G. González, and Y. B., "Physicochemical and microtextural characterization of activated carbons produced from water steam activation of three bamboo species," Journal of Analytical and Applied Pyrolysis, vol. 99, pp. 32-39, 2013
Y. Zhao, F. Fang, H-M. Xiao, Q-P. Feng, L-Y. Xiong, and S-Y. Fu, "Preparation of pore-size controllable activated carbon fibers from bamboo fibers with superior performance for xenon storage," Chemical Engineering Journal, vol. 270, pp. 528-34, 2015.
K. Y. Foo and B. H. Hameed, "Coconut husk derived activated carbon via microwave induced activation: effects of activation agents, preparation parameters and adsorption performance," Chemical Engineering Journal, vol. 184, pp. 57-65, 2012.
J. Jjagwe, P. W. Olupot, E. Menya, and H. M. Kalibbala, "Synthesis and application of granular activated carbon from biomass waste materials for water treatment: A review," Journal of Bioresources and Bioproducts, vol. 6, pp. 292-322, 2021.
V. Gupta, O. Moradi, I. Tyagi, S. Agarwal, H. Sadegh, R. Shahryari-Ghoshekandi, A. Makhlouf, M. Goodarzi, and A. Garshasbi, "Study on the removal of heavy metal ions from industry waste by carbon nanotubes: effect of the surface modification: a review," Critical Reviews in Environmental Science and Technology, vol. 46, pp. 93-118, 2016.
B. Hayati, and N. M. Mahmoodi, "Modification of activated carbon by the alkaline treatment to remove the dyes from wastewater: mechanism, isotherm and kinetic," Desalination and Water Treatment, vol. 47, pp. 322-333, 2012.
M. Sultana, M. H. Rownok, M. Sabrin, M. H. Rahaman, and S. M. Nur Alam, "A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption," Cleaner Engineering and Technology, vol. 6: 100382, pp. 1-14, 2022.
M. Liu, and C. Xiao, "Research progress on modification of activated carbon," E3S web of conferences, 4th International Conference on Energy Materials and Environment Engineering (ICEMEE 2018), vol. 38, no. 02005, pp. 1-4, 2018.
A. E. Ismanto, S. Wang, F. E. Soetaredjo, and S. Ismadji, "Preparation of capacitor’s electrode from cassava peel waste," Bioresource Technology, vol. 101, pp. 3534-3540, 2010.
K. S. W. Sing, and R. T. Williams, "Physisorption hysteresis loops and the characterization of nonporous materials," Adsorption Science & Technology, vol. 22 pp. 773-782, 2004.
D. R. Lobato-Peralta, E. Duque-Brito, A. Ayala-Cort´es, D. M. Arias, A. Longoria, A. K. Cuentas-Gallegos, P.J. Sebastian, and P. U. Okoye, "Advances in activated carbon modification, surface heteroatom configuration, reactor strategies, and regeneration methods for enhanced wastewater treatment," Journal of Environmental Chemical Engineering, vol. 9, pp. 105626, 2021.
S. M. Abegunde, K. S. Idowu, O. M. Adejuwon, and T. Adeyemi-Adejolu, "A review on the influence of chemical modification on the performance of adsorbents," Resources, Environment and Sustainability, vol. 1, p. 100001, 2020.
A. Dąbrowski, P. Podkościelny, Z. Hubicki, and M. Barczak, "Adsorption of phenolic compounds by activated carbon - A critical review," Chemosphere, vol. 58, pp. 1049-1070, 2005.
A. L. Paredes-Doig, M. D. R. Sun-Kou, G. Picasso-Escobar, and J. L. Cannata, "A study of the adsorption of aromatic compounds using activated carbons prepared from chestnut shell," Adsorption Science & Technology, vol. 32, pp. 165-180, 2014.
G. M. S. ElShafei, I. M. A. ElSherbiny, A. S. Darwish, and C. A. Philip, "Artichoke as a non-conventional precursor for activated carbon: Role of the activation process," Journal of Taibah University for Science, vol. 11, pp. 677-688, 2017.
J. Saleem, U. Shahid, M. Hijab, H. Mackey, and G. McKay, "Production and applications of activated carbons as adsorbents from olive stones," Biomass Conversion and Biorefinery, vol. 9, pp. 775-802, 2019.
M. M. Sabzehmeidani, S. Mahnaee, M. Ghaedi, H. Heidari, and V. A. L. Roy, "Carbon based materials: a review of adsorbents for inorganic and organic compounds," Materials Advances, vol. 2, pp. 598-627, 2021.
M. Shaker, A. A. S. Ghazvini, W. Cao, R. Riahifar, and Q. Ge, "Biomass-derived porous carbons as supercapacitor electrodes – A review," New Carbon Materials, vol. 36, pp. 546-572, 2021.
Z. E. Tang, S. Lim, Y. L. Pang, S. H. Shuit, and H. C. Ong, "Utilisation of biomass wastes based activated carbon supported heterogeneous acid catalyst for biodiesel production," Renewable Energy, vol. 158, pp. 91-102, 2020.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












