Physico-chemical characterization of snail shells powder prepared by mechanochemical processes and thermal treatment
DOI:
https://doi.org/10.55713/jmmm.v33i2.1700Keywords:
Snail shell, Thermal treatment, DSC, X-RD, FT-IR, SEM-EDXSAbstract
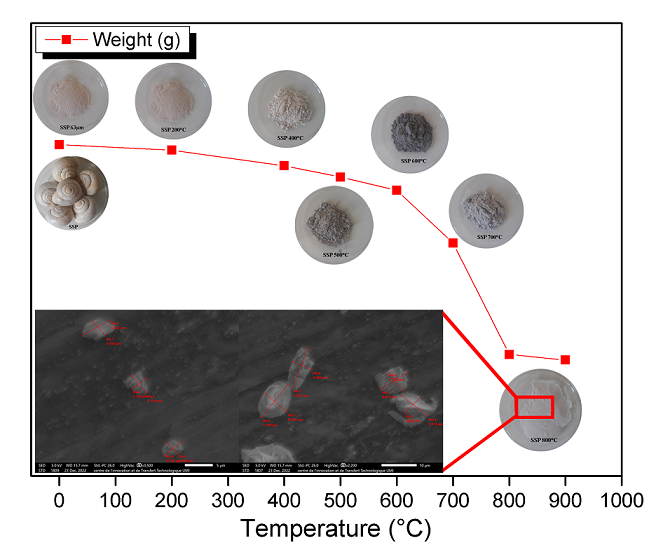
Natural particles are the most abundant resources exist in nature. Bio-sources of CaCO3 particles have attracted the attention of researchers for multiple cosmetics, industrial, and medical applications. This work investigates the structural evolution of CaCO3 containing in snail shell particles prepared by a mechanochemical process using methods of characterization as well as Differential scanning calorimeter (DSC), Thermogravimetric analysis (TGA), X-ray diffraction (X-RD), Fourier transformation infra-red (FT-IR), and Scanning microscopy equipped with Energy-dispersive X-Ray spectroscopy (SEM-EDXS). The result obtained from the above analysis indicates that SSP calcined between 200℃ to 400℃ undergoes an elimination of water molecules, followed by a phase transformation from Aragonite to CaCO3 Calcite. At 800℃, the SSP decomposes CaCO3, giving rise to calcium oxide crystals CaO, which release CO2 molecules. These eliminations and transformations represent a loss of 47.08% of the initial mass at 800℃. The morphological analysis shows the surface of SSP calcined at 800℃ with CaO/CaCO3 crystal formation. Also, the mechanochemical process leads to obtaining an SSP with a size between 3.311 µm to 10.140 µm. Snail shells can be a natural source of CaCO3 and CaO, thanks to their ease of extraction and processing.
Downloads
References
J. Ihli, Y. Y. Kim, E. H. Noel, and F. C. Meldrum, “The effect of additives on amorphous calcium carbonate (ACC): Janus behavior in solution and the solid state,” Advanced Functional Materials, vol. 23, no. 12, pp. 1575-1585, 2013.
B. Janković, I. Smičiklas, N. Manić, A. Mraković, M. Mandić, D. Veljović, and M. Jović, “Thermo-oxidative evolution and physico-chemical characterization of seashell waste for application in commercial sectors,” Thermochimica Acta, vol. 686, p. 178568, 2020.
S. Rujitanapanich, P. Kumpapan, and P. Wanjanoi, “Synthesis of hydroxyapatite from oyster shell via precipitation,” in Energy Procedia, vol. 56, no. C, pp. 112-117, 2014.
T. Witoon, “Characterization of calcium oxide derived from waste eggshell and its application as CO2 sorbent,” Ceramics International, vol. 37, no. 8, pp. 3291-3298, 2011.
M. Egerić, I. Smičiklas, A. Mrakovic, M. Jovic, M. Sljivic-Ivanovic, J. Sokolović, and M. Ristić, “Separation of Cu(II) ions from synthetic solutions and wastewater by raw and calcined seashell waste,” Desalination Water Treat, vol. 132, pp. 205-214, 2018.
E. O. Jatto, I. O. Asia, F. Egharevba, and C. J. Ewansiha, “Kinetics studies of the treatment of wastewater from brewery industry, using powdered snail shell,” Water-Energy Nexus, vol. 3, pp. 95-102, 2020.
O. J. Gbadeyan, S. Adali, G. Bright, and B. Sithole, “The investigation of reinforcement properties of nano-CaCO3 synthesized from Achatina fulica snail shell through mechanochemical methods on epoxy nanocomposites,” Nanocomposites, vol. 7, no. 1, pp. 79-86, 2021.
R. Bhoopathi and M. Ramesh, “Influence of eggshell nanoparticles and effect of alkalization on characterization of industrial hemp fibre reinforced epoxy composites,” Journal of Polymers and the Environment, vol. 28, no. 8, pp. 2178-2190, 2020.
I. Y. Suleiman, V. S. Aigbodion, C. O. Obayi, and K. Mu'azu, “Surface characterisation, corrosion and mechanical properties of polyester-polyester/snail shell powder coatings of steel pipeline for naval applications,” International Journal of Advanced Manufacturing Technology, vol. 101, no. 9-12, pp. 2441-2447, 2019.
C. Wang, J. Zhao, X. Zhao, H. Bala, and Z. Wang, “Synthesis of nanosized calcium carbonate (aragonite) via a polyacrylamide inducing process,” Powder Technology, vol. 163, no. 3, pp. 134-138, 2006.
R. Ouafi, A. Ibrahim, I. Mehdaoui, M. Asri, M. Taleb, and Z. Rais, “Spectroscopic Analysis of chemical compounds derived from the calcination of snail shells waste at different temperatures,” Chemistry Africa, vol. 4, no. 4, pp. 923-933, 2021.
S. Parveen, A. Chakraborty, D. K. Chanda, S. Pramanik, A. Barik, and G. Aditya, “Microstructure analysis and chemical and mechanical characterization of the shells of three freshwater snails,” ACS Omega, vol. 5, no. 40, pp. 25757-25771, 2020.
O. J. Gbadeyan, S. Adali, G. Bright, B. Sithole, and S. Onwubu, “Optimization of Milling Procedures for Synthesizing Nano-CaCO3from Achatina fulica Shell through Mechanochemical Techniques,” Journal of Nanomaterials, vol. 2020, pp. 1-9, 2020.
M. Karaoui, R. Hsissou, M. Alami, and M. Assouag, “Thermal, flow, and mechanical properties of composites based on polystyrene (PS) and snail shell powder (SSP) biofiller (PS/SSP),” Iranian Polymer Journal (English Edition), vol. 32, no. 4, pp. 1-11, 2023.
A. Oyetunji, R. Umunakwe, B. O. Adewuyi, U. S. Nwigwe, and I. J. Umunakwe, “Evaluating the properties of nanoparticles of calcium carbonate obtained from the shells of african giant land snails (Achatina achatina) via in situ deposition technique,” UPB Scientific Bulletin, Series B: Chemistry and Materials Science, vol. 81, no. 1, pp. 86-94, 2019.
P. Maravelaki-Kalaitzaki, A. Bakolas, and A. Moropoulou, “Physico-chemical study of Cretan ancient mortars,” Cement Concrete Research, vol. 33, no. 5, pp. 651-661, 2003.
P. Maravelaki-Kalaitzaki, A. Bakolas, I. Karatasios, and V. Kilikoglou, “Hydraulic lime mortars for the restoration of historic masonry in Crete,” Cement Concrete Research, vol. 35, no. 8, pp. 1577-1586, 2005.
S. Yoshioka, and Y. Kitano, “Transformation of aragonite to calcite through heating,” Geochemical Journal, vol. 19, no. 4, p. 245-249, 1985.
A. Shafiu Kamba, M. Ismail, T. A. Tengku Ibrahim, and Z. A. B. Zakaria, “Synthesis and characterisation of calcium carbonate aragonite nanocrystals from cockle shell powder (Anadara granosa),” Journal of Nanomaterials, vol. 2013, no. 8, p. 5, 2013.
V. Fombuena, L. Bernardi, O. Fenollar, T. Boronat, and R. Balart, “Characterization of green composites from biobased epoxy matrices and bio-fillers derived from seashell wastes,” Materials and Design, vol. 57, pp. 168-174, 2014.
S. Hu, Y. Wang, and H. Han, “Utilization of waste freshwater mussel shell as an economic catalyst for biodiesel production,” Biomass Bioenergy, vol. 35, no. 8, pp. 3627-3635, 2011.
F. Cestari, G. Chemello, A. Galotta, and V. M. Sglavo, “Low-temperature synthesis of nanometric apatite from biogenic sources,” Ceramics Internation, vol. 46, no. 15, 2020.
S. A. S. Bonou, E. Sagbo, C. Aubry, C. Charvillat, B. Ben-Nissan, and S. Cazalbou, “Conversion of snail shells (Achatina achatina) acclimatized in Benin to calcium phosphate for medical and engineering use,” Journal of the Australian Ceramic Society, vol. 55, no. 4, pp. 1177-1186, 2019.
Y. Kezuka, K. Kawai, K. Eguchi, and M. Tajika, “Fabrication of single-crystalline calcite needle-like particles using the aragonite-calcite phase transition,” Minerals, vol. 7, no. 8, p. 133, 2017.
D. Asmi, and A. Zulfia, “Blood cockle shells waste as renewable source for the production of biogenic CaCO3 and Its characterization,” in IOP Conference Series: Earth and Environmental Science, vol. 94, no. 1, p. 012049, 2017.
C. W. Loy, K. A. Matori, W. F. Lim, S. Schmid, N. Zainuddin, Z. A. Wahab, Z. N. Alassan, and M. H. Zaid, “Effects of calcination on the crystallography and nonbiogenic aragonite formation of ark clam shell under ambient condition,” Advances in Materials Science and Engineering, vol. 2016, no. 3-4, pp. 1-8, 2016.
S. Milano, and G. Nehrke, “Microstructures in relation to temperatureinduced aragonite-To-calcite transformation in the marine gastropod Phorcus turbinatus,” Plos One Journal, vol. 13, no. 10, 2018.
T. Laonapakul, R. Sutthi, P. Chaikool, Y. Mutoh, and P. Chindaprasirt, “Optimum conditions for preparation of bio-calcium from blood cockle and golden apple snail shells and characterization,” Journal of The Science Society of Thailand, vol. 45, no. 1, p. 10, 2019.
C. S. Korach, and R. L. Pai, “Mechanical properties of a nano-tructured poly (KAMPS)/aragonite composite,” Mechanics of Biological System and Materials, vol. 2, pp. 131-136, 2011.
M. B. Toffolo, and E. Boaretto, “Nucleation of aragonite upon carbonation of calcium oxide and calcium hydroxide at ambient temperatures and pressures: A new indicator of fire-related human activities,” Journal of Archaeological Science, vol. 49, no. 1, 2014.
L. S. Gomez-Villalba, P. López-Arce, M. Alvarez De Buergo, and R. Fort, “Atomic defects and their relationship to aragonite- calcite transformation in portlandite nanocrystal carbonation,” Crystal Growth and Design, vol. 12, no. 10, 2012.
S. L. M. A. Ghafar, M. Z. Hussein, and Z. A. B. Zakaria, “Synthesis and characterization of cockle shell-based calcium carbonate aragonite polymorph nanoparticles with surface functionalization,” Journal of Nanomaterials, vol. 2017, 2017.
K. N. Islam, M. Z. B. A. Bakar, M. M. Noordin, M. Z. bin Hussein, N. S. B. A. Rahman, and M. E. Ali, “Characterisation of calcium carbonate and its polymorphs from cockle shells (Anadara granosa),” Powder Technology, vol. 213, no. 1, 2011.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












