Leonardite humic acid activated carbon/MnO\(_{2}\) composite nanostructures for supercapacitors
DOI:
https://doi.org/10.55713/jmmm.v34i2.1932Keywords:
Leonardite humic acid, Activated carbon, MnO2, Electrochemical properties, SupercapacitorsAbstract
Abstract
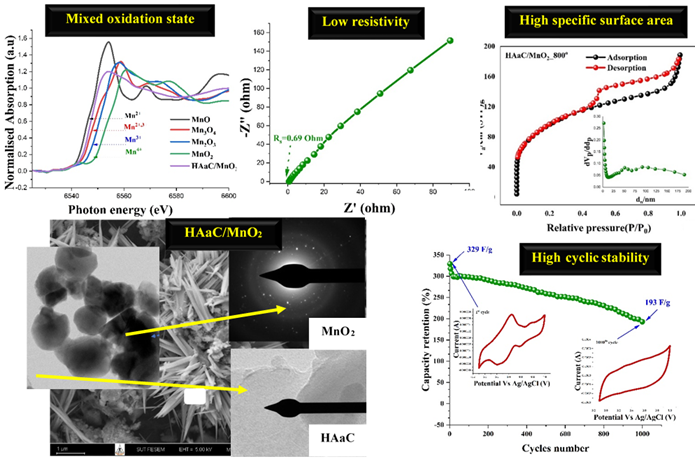
This work reports the preparation and electrochemical studies of activated carbon derived from leonardite humic acid composited with MnO2 for supercapacitors. Activated carbon contains high conductivity, high specific surface area, and accommodates large volume expansion/contraction during charging/discharging process. Meanwhile, MnO2 has very high theoretical specific capacity (1370 F∙g‒1). Their composite could significantly improve both the storage performance and cycle stability of supercapacitors. Moreover, humic acid from leonardite was selected to add value to this waste and reduce environmental pollution. By varying the carbonization temperature (500℃ to 800℃), the prepared samples carbonized at 800℃ exhibited fascinating properties. The oxidation state of Mn ions was in the mixed state of Mn+2 (41.2%) and Mn+2, +3 (52.8%). A gravimetric capacitance of 329 F∙g‒1 and 294 F∙g‒1 were observed at 2 mVs-1 and 0.5 Ag-1, respectively. The remaining gravimetric capacitance of 193 F∙g‒1 was evaluated at 1000 cycles, indicating its high cycle performance. Moreover, the gravimetric energy of 37.51 Wh∙kg‒1 and gravimetric power of 272.96 W∙kg‒1 were observed. When combined, the interesting electrochemical properties of leonardite humic acid-activated carbon/MnO2 composite nanostructures make them important options for supercapacitor application.
Downloads
References
D. Salinas-Torres, R. Ruiz-Rosas, E. Morallón, and D, Cazorla-Amorós, “Strategies to enhance the performance of electrochemical capacitors based on carbon materials,” Frontiers in Materials, vol. 6, pp. 115, 2019. DOI: https://doi.org/10.3389/fmats.2019.00115
M. Shahedi Asl, R. Hadi, L. Salehghadimi, A. Goljanian Tabrizi, S. Farhoudian, A. Babapoor, M. Pahlevani, “Flexible all-solid-state supercapacitors with high capacitance, long cycle life, and wide operational potential window: recent progress and future perspectives,” Journal of Energy Storage, vol. 50, pp. 104223, 2022. DOI: https://doi.org/10.1016/j.est.2022.104223
J. W. Long, D. B´elanger, T. Brousse, W. Sugimoto, M. B. Sassin, and O. Crosnier, “Asymmetric electrochemical capacitors stretching the limits of aqueous electrolytes,” MRS Bulletin, vol. 36, pp. 513-522, 2011. DOI: https://doi.org/10.1557/mrs.2011.137
Y. Huang, J. Liang, and Y. Chen, “An overview of the applications of graphene-based materials in supercapacitors,” Small, vol. 8, pp. 1805-1834, 2012. DOI: https://doi.org/10.1002/smll.201102635
A. Borenstein, O. Hanna, R. Attias, S. Luski, T. Brousse, and D. Aurbach, “Carbon-based composite materials for supercapacitor electrodes: A review,” Journal of Materials Chemistry, vol 5, pp. 12653-12672, 2017. DOI: https://doi.org/10.1039/C7TA00863E
M. Liang, and H. Xin, “Microwave to terahertz: characterization of carbon-based nanomaterials,” IEEE Microwave Magazine, vol. 15, pp. 40-51, 2015. DOI: https://doi.org/10.1109/MMM.2013.2288708
S. Tajik, D. P. Dubal, P. Gomez-Romero, A. Yadegari, A. Rashidi, B. Nasernejad, Inamuddin, A. M. Asiri, “Nanostructured mixed
transition metal oxides for high performance asymmetric supercapacitors: facile synthetic strategy,” International Journal of Hydrogen Energy, vol. 42, pp. 12384-12395, 2017. DOI: https://doi.org/10.1016/j.ijhydene.2017.03.117
B. E. Conway. Electrochemical Supercapacitor: Scientific Fundamentals and Technological Applications. New York: Kluwer Academic/Plenum Publishers, 1999.
S. Schrade, Z. Zhao, Z. Supiyeva, X. Chen, S. Dsoke, and Q. Abbas, “An asymmetric MnO2 activated carbon super-capacitor with highly soluble choline nitrate-based aqueous electrolyte for sub-zero temperatures,” Electrochimica Acta, vol. 425, pp. 140708, 2022. DOI: https://doi.org/10.1016/j.electacta.2022.140708
L. H. Tseng, C. H. Hsiao, D. D. Nguyen, P. Y Hsieh, C. Y. Lee, and N-H. Tai, “Activated carbon sandwiched manganese dioxide/graphene ternary composites for supercapacitor electrodes,” Electrochimica Acta, vol. 266, pp. 218-292, 2018. DOI: https://doi.org/10.1016/j.electacta.2018.02.029
Y. Xie, C. Yang, P. Chen, D. Yuan, and K. Guo, “MnO2-decorated hierarchical porous carbon composites for high-performance asymmetric supercapacitors,” Journal of Power Sources, vol. 425, pp. 1-9, 2019.
S. Barbato, and J. L. Gautier, “Hollandite cathodes for lithium ion batteries: Thermodynamic and kinetics studies of lithium insertion into BaMMn7O16 (M=Mg, Mn, Fe, Ni),” Electro-chimica Acta, vol. 46, no. 18, pp. 2767-2776, 2001. DOI: https://doi.org/10.1016/S0013-4686(01)00506-0
A. David, M. Tompsett, and S. Islam, “Electrochemistry of Hollandite α-MnO : Li-Ion and Na-Ion Incorporation,” Chemistry of Materials, vol. 25, no. 12, pp. 2515-2526, 2013. DOI: https://doi.org/10.1021/cm400864n
J. G. Wang, F. Kang, and B. Wei,” Engineering of MnO2-based nanocomposites for high performance supercapacitors,” Progress in Materals Science, vol. 74, pp. 51-124, 2015. DOI: https://doi.org/10.1016/j.pmatsci.2015.04.003
R. P. Schwarzenbach, P. M. Gschwend, and D. M. Imboden, 1993. Environmental organic Chemistry. New Youk: John Wiley & Sons Inc.
E. A. Ghabbour and G. Davies, Humic Substances: Structures, Properties and Uses. Cambridge: Special publication, 1998.
S. Ozuzun, and B. Uzal, “Performance of leonardite humic acid as a novel superplasticizer in Portland cement systems,” Journal of Building. Engineering, vol. 42, pp. 103070, 2021. DOI: https://doi.org/10.1016/j.jobe.2021.103070
B. A. G de Melo, F. L. Motta, and M. H. A. Santana, “ Humic acids: Structural properties and multiple functionalities for novel technological developments,” Materials Science and Engineering C, vol. 62, pp. 967-974, 2016. DOI: https://doi.org/10.1016/j.msec.2015.12.001
J. Luo, H. T. Zhu, H. M. Fan, J. K. Liang, H. L. Shi, G. H. Rao, J. B. Li, Z. M. Du, and Z. X. Shen, “Synthesis of single-crystal tetragonal α-MnO2 nanotubes,” Journal of. Physical Chemistry C, vol. 112, pp. 12594-12598, 2008. DOI: https://doi.org/10.1021/jp8052967
O. Canieren, C. Karaguzel, and A. Aydin, “Effect of physical pre-enrichment on humic substance recovery from leonardite,” Physicochemical. Problems of Mineral Processing, vol. 53, no. 1, pp. 502-514, 2017.
S. Sudiono, M. Yuniarti, D. Siswanta, and E. Kunarti, “The role of carboxyl and hydroxyl groups of humic acid in removing AuCl4 from aqueous solution,” Indian Journal of Chemistry, vol. 17, no.1, pp. 95, 2017. DOI: https://doi.org/10.22146/ijc.23620
M. Králik, “Adsorption, chemisorption, and catalysis,” Chemical Papers, vol. 68, no. 12, pp. 1625-1638, 2014. DOI: https://doi.org/10.2478/s11696-014-0624-9
D. Wu, X. Xie, Y. Zhang, D. Zhang, W. Du, X. Zhang, and B. Wang, “MnO2/carbon composites for supercapacitor: Synthesis and electrochemical performance,” Frontiers in Materials, vol. 7, pp. 2, 2020. DOI: https://doi.org/10.3389/fmats.2020.00002
X. Wang, D. Wu, X. Song, W. Du, X. Zhao, and D. Zhang, “Review on carbon/polyaniline hybrids: design and synthesis for supercapacitor,” Molecules, vol. 24, pp. 2263, 2019. DOI: https://doi.org/10.3390/molecules24122263
S. Nilmoung, W. Limphirat, and S. Maensiri, “Electrochemical properties of ACNF/Li2FeSiO4 composite nanostructures for supercapacitors,” Journal of Alloys and Compounds, vol. 907, pp. 164466, 2022. DOI: https://doi.org/10.1016/j.jallcom.2022.164466
M. Fu, Z. Zhu, Z. Zhang, Q. Zhuang, F. Gao, W. Chen, H. Yu, and Q. Liu, “Microwave assisted growth of MnO2 on biomass carbon for advanced supercapacitor electrode materials,” Journal of Materials Science, vol. 56, pp. 6987-6996, 2021. DOI: https://doi.org/10.1007/s10853-020-05723-y
S. Nilmoung, T. Sinprachim, I. Kotutha, P. Kidkhunthod, R. Yimnirun, S. Rujirawat, and S. Maensiri, “Electrospun carbon/ CuFe2O4 composite nanofibers with improved electrochemical energy storage performance,”Journal of Alloys and Compounds, vol. 688, pp. 1131-1140, 2016. DOI: https://doi.org/10.1016/j.jallcom.2016.06.251
X. Wang, J. Chu, H. J. Yan, and H. K. Zhang, “Synthesis and characterization of MnO2/Eggplant carbon composite for enhanced supercapacitors,” Heliyon, vol. 82, pp. e10631, 2022. DOI: https://doi.org/10.1016/j.heliyon.2022.e10631
Y. Wang, Q. He, H. Qu, X. Zhang, J. Guo, J. Zhu, G. Zhao, H. A. Colorado, J. Yu, L. Sun, S. Bhana, M.A. Khan, X. Huang, D. P. Young, H. Wang, X. Wang, S. Wei, and Z. Guo, “Magnetic graphene oxide nanocomposites: Nanoparticles growth mechanism and property analysis,” Journal of Materials Chemistry C, vol. 2, pp. 9478-9488, 2014. DOI: https://doi.org/10.1039/C4TC01351D
K. Wang, L. Huang, N. Eedugurala, S. Zhang, M. A. Sabuj, N. Rai, X. Gu, J. D. Azoulay, and T. N. Ng, “Wide potential window supercapacitors using open-shell donor–acceptor conjugated polymers with stable n-doped states,” Advanced Energy Materials, vol. 9, pp. 1-8, 2019. DOI: https://doi.org/10.1002/aenm.201902806
X. Liu, L. Zhou, Y. Zhao, L. Bian, X. Feng, and Q. Pu, “Spherical nitrogen-rich porous carbon shells obtained from a porous organic framework for the supercapacitor,” ACS Applied Materials & Interfaces, vol. 5, pp. 10280-10287, 2013. DOI: https://doi.org/10.1021/am403175q
Y. Zhao, H. Hao, T. Song, X. Wang, C. Li, and W. Li, “MnO2-graphene based composites for supercapacitors: Synthesis, performance and prospects” Journal of Alloys and Compounds, vol. 914, pp. 165343, 2022. DOI: https://doi.org/10.1016/j.jallcom.2022.165343
Y. Xie, C. Yang, P. Chen, D. Yuan, and K. Guo, “MnO2-decorated hierarchical porous carbon composites for high-performance asymmetric,” Journal of Power Sources, vol. 425, pp. 1-9, 2019. DOI: https://doi.org/10.1016/j.jpowsour.2019.03.122
Z. H. Huang, Y. Song, D. Y. Feng, Z. Sun, X. Sun, and X. X. Liu, “High mass loading MnO2 with hierarchical nanostructures for supercapacitors,” ACS Nano, vol. 12, pp. 3557-3567, 2018. DOI: https://doi.org/10.1021/acsnano.8b00621
H. Xu, X. Hu, H. Yang, Y. Sun, C. Hu, and Y. Huang, “Flexible asymmetric micro-supercapacitors based on Bi2O3 and MnO2 nanoflowers: Larger areal mass promises higher energy density,” Advanced Energy Materials, vol. 5, pp. 1401882, 2015. DOI: https://doi.org/10.1002/aenm.201401882
X. Li, Y. Tang, C. Han, Z. Wei, H. Fan, H. Lv, T. Cai, Y. Cui, W. Xing, Z. Yan, C. Zhi, and H. Li, “A static tin-manganese battery with 30000-cycle lifespan based on stabilized Mn3+/ Mn2+ redox chemistry,” ACS Nano, vol. 17, no. 5, pp. 5083-5094, 2023. DOI: https://doi.org/10.1021/acsnano.3c00242
C. Zhan, J. Lu, A. J. Kropf, T. Wu. A. N. Jansen, J. K. Sun, X. Qiu, and K. Amine, “Mn (II) deposition on anodes and its effects on capacity fade in spinel lithium manganate-carbon systems,” Nature Communications, vol. 4, pp. 2437, 2013. DOI: https://doi.org/10.1038/ncomms3437
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












