Amino-containing polymer-coated magnetite nanoparticles as nano-adsorbents for bisphenol A: Synthesis, kinetic and thermodynamic study
DOI:
https://doi.org/10.55713/jmmm.v32i2.1261Keywords:
Magnetite, Nanoparticle, Bisphenol A, Nano-adsorbent, AdsorptionAbstract
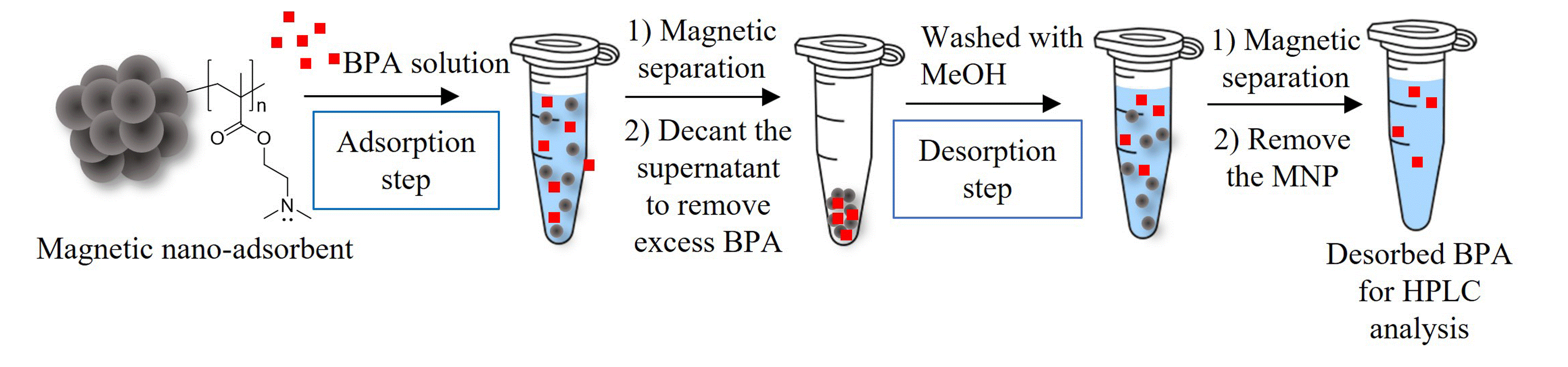
Magnetite nanoparticles coated with poly(dimethyl aminoethyl methacrylate) (PDMAEMA@MNPs) and their quarternized form (PQDMAEMA@MNPs) were successfully synthesized and used as nano-adsorbents for bisphenol A (BPA). The particles were spherical with the average particle size between 10 and 20 nm in diameter with a moderate degree of nanoclustering (ca.150-200 particles/cluster). In terms of adsorption properties, the PDMAEMA@MNPs exhibited a higher BPA adsorption capacity (1.05 mg/g MNP at pH 9) than the quaternized form (0.50 mg/g MNP at pH 9). Equilibrium isotherm, kinetic, and thermodynamic characteristics of BPA adsorption on the PDMAEMA@MNPs were investigated. It was found that the BPA adsorption on the MNPs reached an equilibrium within 5 min and the maximum adsorption capacity (qe) was 9.88 mg/g. The adsorption isotherm study results indicated that the BPA adsorption process on PDMAEMA@MNPs exhibited the best fit with the Freundlich model, and the adsorption kinetics followed the pseudo-second order model with the R2 value of 1.00. The thermodynamic data exhibited a negative enthalpy change (ΔH◦ = -2585.571 J/mol), indicating exothermic BPA adsorption, and a negative Gibbs free energy (ΔG◦), implying a spontaneous BPA adsorption process.
Downloads
References
L. Cui, J. Wei, X. Du, and X. Zhou, "Preparation and evaluation of self-assembled porous microspheres–fibers for removal of bisphenol A from aqueous solution," Industrial & engineering chemistry research, vol. 55, no. 6, pp. 1566-1574, 2016. DOI: https://doi.org/10.1021/acs.iecr.5b04306
H. Xiong, L. Guo, X. Mao, T. Tan, H. Wan, and Y. Wan, "A magnetic hydrophilic molecularly imprinted material with multiple stimuli-response properties for efficient recognition of bisphenol A in beverages," Food Chemistry, vol. 331, p. 127311, 2020. DOI: https://doi.org/10.1016/j.foodchem.2020.127311
J. H. Kang, D. Aasi, and Y. Katayama, "Bisphenol A in the aquatic environment and its endocrine-disruptive effects on aquatic organisms," Critical reviews in toxicology, vol. 37, no. 7, pp. 607-625, 2007. DOI: https://doi.org/10.1080/10408440701493103
M. Martín-Lara, M. Calero, A. Ronda, I. Iáñez-Rodríguez, and C. Escudero, "Adsorptive behavior of an activated carbon for bisphenol A removal in single and binary (bisphenol A—heavy metal) solutions," Water, vol. 12, no. 8, p. 2150, 2020. DOI: https://doi.org/10.3390/w12082150
K. Ragavan and N. K. Rastogi, "β-Cyclodextrin capped graphene-magnetite nanocomposite for selective adsorption of Bisphenol-A," Carbohydrate polymers, vol. 168, pp. 129-137, 2017. DOI: https://doi.org/10.1016/j.carbpol.2017.03.045
D. Balarak, F. K. Mostafapour, S. M. Lee, and C. Jeon, "Adsorption of bisphenol a using dried rice husk: equilibrium, kinetic and thermodynamic studies," Applied Chemistry for Engineering, vol. 30, no. 3, pp. 316-323, 2019.
S. Rovani, J. J. Santos, S. N. Guilhen, P. Corio, and D. A. Fungaro, "Fast, efficient and clean adsorption of bisphenol-A using renewable mesoporous silica nanoparticles from sugarcane waste ash," RSC Advances, vol. 10, no. 46, pp. 27706-27712, 2020. DOI: https://doi.org/10.1039/D0RA05198E
X. Zhou, J. Wei, K. Liu, N. Liu, and B. Zhou, "Adsorption of bisphenol A based on synergy between hydrogen bonding and hydrophobic interaction," Langmuir, vol. 30, no. 46, pp. 13861-13868, 2014. DOI: https://doi.org/10.1021/la502816m
M. A. Zazouli, F. Veisi, and A. Veisi, "Modeling of Bisphenol A (BPA) Removal from Aqueous Solutions by Adsorption Using Response Surface Methodology (RSM)," International Journal of Chemical and Molecular Engineering, vol. 10, no. 2, pp. 228-233, 2016.
Q. Li et al., "Enhanced adsorption of bisphenol A from aqueous solution with 2-vinylpyridine functionalized magnetic nanoparticles," Polymers, vol. 10, no. 10, p. 1136, 2018. DOI: https://doi.org/10.3390/polym10101136
H. Abdullah, M. Nuid, N. A. A. Salim, N. A. Zainuddin, and N. Ahmad, "Bisphenol A Removal by Adsorption Using Waste Biomass: Isotherm and Kinetic Studies," Biointerface Research in Applied Chemistry, vol. 11, no. 1, pp. 8467-8481, 2021. DOI: https://doi.org/10.33263/BRIAC111.84678481
B. Orimolade, F. Adekola, and G. Adebayo, "Adsorptive removal of bisphenol A using synthesized magnetite nanoparticles," Applied water science, vol. 8, no. 1, pp. 1-8, 2018. DOI: https://doi.org/10.1007/s13201-018-0685-y
M. B. Ahmed, J. L. Zhou, H. H. Ngo, W. Guo, N. S. Thomaidis, and J. Xu, "Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: a critical review," Journal of hazardous materials, vol. 323, pp. 274-298, 2017. DOI: https://doi.org/10.1016/j.jhazmat.2016.04.045
D. Bing-zhi, C. Hua-qiang, W. Lin, X. Sheng-ji, and G. Nai-yun, "The removal of bisphenol A by hollow fiber microfiltration membrane," Desalination, vol. 250, no. 2, pp. 693-697, 2010. DOI: https://doi.org/10.1016/j.desal.2009.05.022
J. Wang and M. Zhang, "Adsorption characteristics and mechanism of bisphenol A by magnetic biochar," International journal of environmental research and public health, vol. 17, no. 3, p. 1075, 2020. DOI: https://doi.org/10.3390/ijerph17031075
M. El-Bindary, M. El-Desouky, and A. El-Bindary, "Adsorption of industrial dye from aqueous solutions onto thermally treated green adsorbent: A complete batch system evaluation," Journal of Molecular Liquids, vol. 346, p. 117082, 2022. DOI: https://doi.org/10.1016/j.molliq.2021.117082
K. Elwakeel, A. El-Bindary, and E. Kouta, "Retention of copper, cadmium and lead from water by Na-Y-Zeolite confined in methyl methacrylate shell," Journal of environmental chemical engineering, vol. 5, no. 4, pp. 3698-3710, 2017. DOI: https://doi.org/10.1016/j.jece.2017.06.049
H. A. Kiwaan, T. M. Atwee, E. A. Azab, and A. A. El‐Bindary, "Efficient photocatalytic degradation of Acid Red 57 using synthesized ZnO nanowires," Journal of the Chinese Chemical Society, vol. 66, no. 1, pp. 89-98, 2019. DOI: https://doi.org/10.1002/jccs.201800092
N. Hassan, A. Shahat, A. El-Didamony, M. El-Desouky, and A. El-Bindary, "Synthesis and characterization of ZnO nanoparticles via zeolitic imidazolate framework-8 and its application for removal of dyes," Journal of molecular structure, vol. 1210, p. 128029, 2020. DOI: https://doi.org/10.1016/j.molstruc.2020.128029
Y. Su et al., "Extraction and detection of bisphenol A in human serum and urine by aptamer-functionalized magnetic nanoparticles," Analytical and bioanalytical chemistry, vol. 410, no. 7, pp. 1885-1891, 2018. DOI: https://doi.org/10.1007/s00216-017-0801-0
P. Theamdee, B. Rutnakornpituk, U. Wichai, and M. Rutnakornpituk, "Recyclable magnetic nanoparticle grafted with pH-responsive polymer for adsorption with DNA," Journal of nanoparticle research, vol. 16, no. 7, pp. 1-12, 2014. DOI: https://doi.org/10.1007/s11051-014-2494-z
B. Rutnakornpituk, T. Theppaleak, M. Rutnakornpituk, and T. Vilaivan, "Recyclable magnetite nanoparticle coated with cationic polymers for adsorption of DNA," Journal of Biomaterials science, Polymer edition, vol. 27, no. 11, pp. 1200-1210, 2016. DOI: https://doi.org/10.1080/09205063.2016.1189378
N. Deepuppha, S. Khadsai, B. Rutnakornpituk, F. Kielar, and M. Rutnakornpituk, "Reusable pectin‐coated magnetic nanosorbent functionalized with an aptamer for highly selective Hg2+ detection," Polymers for Advanced Technologies, vol. 32, no. 5, pp. 2207-2217, 2021. DOI: https://doi.org/10.1002/pat.5254
X. Wu, Y. Li, X. Zhu, C. He, Q. Wang, and S. Liu, "Dummy molecularly imprinted magnetic nanoparticles for dispersive solid-phase extraction and determination of bisphenol A in water samples and orange juice," Talanta, vol. 162, pp. 57-64, 2017. DOI: https://doi.org/10.1016/j.talanta.2016.10.007
J. Connolly, T. St Pierre, M. Rutnakornpituk, and J. Riffle, "Silica coating of cobalt nanoparticles increases their magnetic and chemical stability for biomedical applications," European Cells and Materials, vol. 3, no. 2, pp. 106-109, 2002.
M. Rutnakornpituk, V. Baranauskas, J. Riffle, J. Connolly, T. St Pierre, and J. Dailey, "Polysiloxane fluid dispersions of Cobalt nanoparticles in silica spheres for use in opthalmic applications," European Cells and Materials, vol. 3, no. 2, pp. 102-105, 2002.
S. Khadsai, B. Rutnakornpituk, T. Vilaivan, M. Nakkuntod, and M. Rutnakornpituk, "Anionic magnetite nanoparticle conjugated with pyrrolidinyl peptide nucleic acid for DNA base discrimination," Journal of Nanoparticle Research, vol. 18, no. 9, pp. 1-15, 2016. DOI: https://doi.org/10.1007/s11051-016-3574-z
B. Rutnakornpituk, U. Wichai, T. Vilaivan, and M. Rutnakornpituk, "Surface-initiated atom transfer radical polymerization of poly (4-vinylpyridine) from magnetite nanoparticle," Journal of Nanoparticle Research, vol. 13, no. 12, pp. 6847-6857, 2011. DOI: https://doi.org/10.1007/s11051-011-0592-8
S. Meerod, B. Rutnakornpituk, U. Wichai, and M. Rutnakornpituk, "Hydrophilic magnetic nanoclusters with thermo-responsive properties and their drug controlled release," Journal of Magnetism and Magnetic Materials, vol. 392, pp. 83-90, 2015. DOI: https://doi.org/10.1016/j.jmmm.2015.05.022
T. Theppaleak, G. Tumcharern, U. Wichai, and M. Rutnakornpituk, "Synthesis of water dispersible magnetite nanoparticles in the presence of hydrophilic polymers," Polymer bulletin, vol. 63, no. 1, pp. 79-90, 2009. DOI: https://doi.org/10.1007/s00289-009-0075-6
N. Deepuppha, A. Thongsaw, B. Rutnakornpituk, W. C. Chaiyasith, and M. Rutnakornpituk, "Alginate-based magnetic nanosorbent immobilized with aptamer for selective and high adsorption of Hg 2+ in water samples," Environmental Science and Pollution Research, vol. 27, no. 11, pp. 12030-12038, 2020. DOI: https://doi.org/10.1007/s11356-020-07809-1
S. Meerod, N. Deepuppha, B. Rutnakornpituk, and M. Rutnakornpituk, "Reusable magnetic nanocluster coated with poly (acrylic acid) and its adsorption with an antibody and an antigen," Journal of Applied Polymer Science, vol. 135, no. 16, p. 46160, 2018. DOI: https://doi.org/10.1002/app.46160
S. Khadsai et al., "Poly (acrylic acid)-grafted magnetite nanoparticle conjugated with pyrrolidinyl peptide nucleic acid for specific adsorption with real DNA," Colloids and Surfaces B: Biointerfaces, vol. 165, pp. 243-251, 2018. DOI: https://doi.org/10.1016/j.colsurfb.2018.02.039
P. Theamdee, R. Traiphol, B. Rutnakornpituk, U. Wichai, and M. Rutnakornpituk, "Surface modification of magnetite nanoparticle with azobenzene-containing water dispersible polymer," Journal of Nanoparticle Research, vol. 13, no. 10, pp. 4463-4477, 2011. DOI: https://doi.org/10.1007/s11051-011-0399-7
M. A. De Jesús‐Téllez, D. M. Sánchez‐Cerrillo, P. Quintana‐Owen, U. S. Schubert, D. Contreras‐López, and C. Guerrero‐Sánchez, "Kinetic Investigations of Quaternization Reactions of Poly [2‐(dimethylamino) ethyl methacrylate] with Diverse Alkyl Halides," Macromolecular Chemistry and Physics, vol. 221, no. 9, p. 1900543, 2020. DOI: https://doi.org/10.1002/macp.201900543
Z. Dong et al., "Synthesis and responsive behavior of poly (N, N-dimethylaminoethyl methacrylate) brushes grafted on silica nanoparticles and their quaternized derivatives," Polymer, vol. 53, no. 10, pp. 2074-2084, 2012. DOI: https://doi.org/10.1016/j.polymer.2012.03.011
A. B. Shatan et al., "Cationic Polymer-Coated Magnetic Nanoparticles with Antibacterial Properties: Synthesis and In Vitro Characterization," Antibiotics, vol. 10, no. 9, p. 1077, 2021. DOI: https://doi.org/10.3390/antibiotics10091077
T. Theppaleak, B. Rutnakornpituk, U. Wichai, T. Vilaivan, and M. Rutnakornpituk, "Magnetite nanoparticle with positively charged surface for immobilization of peptide nucleic acid and deoxyribonucleic acid," Journal of biomedical nanotechnology, vol. 9, no. 9, pp. 1509-1520, 2013. DOI: https://doi.org/10.1166/jbn.2013.1645
F. Adekola, D. Hodonou, and H. Adegoke, "Thermodynamic and kinetic studies of biosorption of iron and manganese from aqueous medium using rice husk ash," Applied Water Science, vol. 6, no. 4, pp. 319-330, 2016. DOI: https://doi.org/10.1007/s13201-014-0227-1
C. A. Dinçer, N. Yıldız, N. Aydoğan, and A. Çalımlı, "A comparative study of Fe3O4 nanoparticles modified with different silane compounds," Applied surface science, vol. 318, pp. 297-304, 2014. DOI: https://doi.org/10.1016/j.apsusc.2014.06.069
P. Jiang et al., "Effect of DMAEMA content and polymerization mode on morphologies and properties of pH and temperature double-sensitive cellulose-based hydrogels," Journal of Macromolecular Science, Part A, vol. 57, no. 3, pp. 207-216, 2020. DOI: https://doi.org/10.1080/10601325.2019.1681899
A. Hernández-Martínez and E. Bucio, "Novel pH-and temperature-sensitive behavior of binary graft DMAEMA/PEGMEMA onto LDPE membranes," Designed monomers and polymers, vol. 12, no. 6, pp. 543-552, 2009. DOI: https://doi.org/10.1163/138577209X12478293300757
G. Estrada-Villegas and E. Bucio, "Temperature-and pH-responsive behavior of a novel copolymer of (PP-g-DMAEMA)-g-AAc," Journal of Radioanalytical and Nuclear Chemistry, vol. 292, no. 1, pp. 1-6, 2012. DOI: https://doi.org/10.1007/s10967-011-1603-z
L. Xu et al., "Pore structure and fractal characteristics of different shale lithofacies in the dalong formation in the western area of the lower yangtze platform," Minerals, vol. 10, no. 1, p. 72, 2020. DOI: https://doi.org/10.3390/min10010072
F. Wang, Q. Zeng, W. Su, M. Zhang, L. Hou, and Z. L. Wang, "Adsorption of bisphenol A on peanut shell biochars: The effects of surfactants," Journal of Chemistry, vol. 2019, 2019. DOI: https://doi.org/10.1155/2019/2428505
Y. Zhang et al., "Recyclable removal of bisphenol A from aqueous solution by reduced graphene oxide–magnetic nanoparticles: adsorption and desorption," Journal of colloid and interface science, vol. 421, pp. 85-92, 2014. DOI: https://doi.org/10.1016/j.jcis.2014.01.022
Y. Li et al., "Novel microporous β-cyclodextrin polymer as sorbent for solid-phase extraction of bisphenols in water samples and orange juice," Talanta, vol. 187, pp. 207-215, 2018. DOI: https://doi.org/10.1016/j.talanta.2018.05.030
C. Cáceres et al., "Molecularly imprinted polymers for the selective extraction of bisphenol a and progesterone from aqueous media," Polymers, vol. 10, no. 6, p. 679, 2018. DOI: https://doi.org/10.3390/polym10060679
G. Zeng et al., "Adsorption behavior of bisphenol A on sediments in Xiangjiang River, Central-south China," Chemosphere, vol. 65, no. 9, pp. 1490-1499, 2006. DOI: https://doi.org/10.1016/j.chemosphere.2006.04.013
N. Genç, Ö. Kılıçoğlu, and A. O. Narci, "Removal of Bisphenol A aqueous solution using surfactant-modified natural zeolite: Taguchi’s experimental design, adsorption kinetic, equilibrium and thermodynamic study," Environmental Technology, vol. 38, no. 4, pp. 424-432, 2017. DOI: https://doi.org/10.1080/21622515.2016.1196739
N. Rahmat, T. Hadibarata, A. Yuniarto, M. Elshikh, and A. Syafiuddin, "Isotherm and kinetics studies for the adsorption of bisphenol A from aqueous solution by activated carbon of Musa acuminata," in IOP Conference Series: Materials Science and Engineering, 2019, vol. 495, no. 1: IOP Publishing, p. 012059. DOI: https://doi.org/10.1088/1757-899X/495/1/012059
K. Y. Foo and B. H. Hameed, "Insights into the modeling of adsorption isotherm systems," Chemical engineering journal, vol. 156, no. 1, pp. 2-10, 2010. DOI: https://doi.org/10.1016/j.cej.2009.09.013
M. H. Dehghani et al., "Adsorptive removal of endocrine disrupting bisphenol A from aqueous solution using chitosan," Journal of Environmental Chemical Engineering, vol. 4, no. 3, pp. 2647-2655, 2016. DOI: https://doi.org/10.1016/j.jece.2016.05.011
K. V. Kumar, "Linear and non-linear regression analysis for the sorption kinetics of methylene blue onto activated carbon," Journal of hazardous materials, vol. 137, no. 3, pp. 1538-1544, 2006. DOI: https://doi.org/10.1016/j.jhazmat.2006.04.036
S. Chowdhury and P. Saha, "Adsorption kinetic modeling of safranin onto rice husk biomatrix using pseudo‐first‐and pseudo‐second‐order kinetic models: Comparison of linear and non‐linear methods," CLEAN–Soil, Air, Water, vol. 39, no. 3, pp. 274-282, 2011. DOI: https://doi.org/10.1002/clen.201000170
G. A. AlHazmi, K. S. AbouMelha, M. G. El-Desouky, and A. A. El-Bindary, "Effective adsorption of doxorubicin hydrochloride on zirconium metal-organic framework: Equilibrium, kinetic and thermodynamic studies," Journal of Molecular Structure, vol. 1258, p. 132679, 2022. DOI: https://doi.org/10.1016/j.molstruc.2022.132679
A. E. Ofomaja, E. B. Naidoo, and A. Pholosi, "Intraparticle diffusion of Cr (VI) through biomass and magnetite coated biomass: A comparative kinetic and diffusion study," South African Journal of Chemical Engineering, vol. 32, no. 1, pp. 39-55, 2020. DOI: https://doi.org/10.1016/j.sajce.2020.01.005
V. A. R. Villegas, J. I. D. L. Ramírez, E. H. Guevara, S. P. Sicairos, L. A. H. Ayala, and B. L. Sanchez, "Synthesis and characterization of magnetite nanoparticles for photocatalysis of nitrobenzene," Journal of Saudi Chemical Society, vol. 24, no. 2, pp. 223-235, 2020. DOI: https://doi.org/10.1016/j.jscs.2019.12.004
Downloads
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












