Study of corrosion properties of carbon steel, 304 and 316L stainless steels in sulfuric acid and their degradation products
DOI:
https://doi.org/10.55713/jmmm.v33i4.1672Keywords:
Sulfuric acid, corrosion properties, carbon steel, SS304, SS316LAbstract
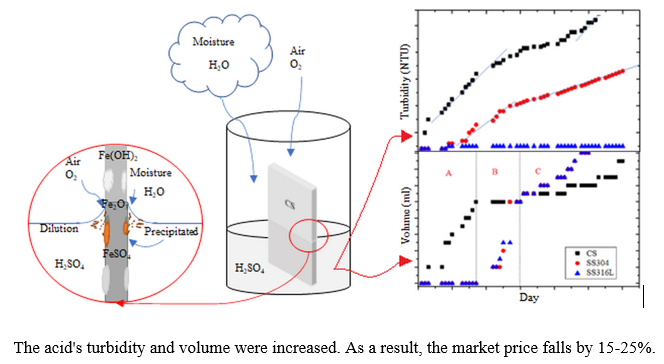
In this study, corrosion properties of carbon steel and stainless steels, and degradation of sulfuric acid by the corrosion mechanism are presented. Carbon steel (CS), 304 stainless steel (SS304), and 316L stainless steel (SS316L) specimens were analyzed through their electrochemical response by using a potentiostat measurement. The specimens were submerged in concentrated 98 wt.% of H2SO4 acid for 0 day to 60 day. The degradation of H2SO4 was determined by its volume change, turbidity, and color due to the corrosion mechanism. Corrosion rate of CS, SS304 and SS316L specimens are 43.237 mm/year, 0.420 mm/year and 0.086 mm/year, respectively. After 60 days, the weight-loss of CS, SS304 and SS316L specimens are 48 wt%, 33 wt% and 0.1 wt%, respectively. Corrosion resistance of the materials are influenced by the passive oxide layer that forms on its surface and associated with electrochemical activity or semiconductive composition. The degradation of H2SO4 acid was observed due to the corrosion process of specimens and related to the turbidity and volume increase while the wt% concentration of H2SO4 acid decreases. In order to make material choices that enable continuous and safe operation of the process, it is important to understand the corrosion mechanism changes.
Downloads
References
Z. Panossian, N. L. Almeida, R. M. R. de Sousa, G. de S. Pimenta, and L. B. S. Marques, “Corrosion of carbon steel pipes and tanks by concentrated sulfuric acid: A review,” Corrosion Science, vol. 74, pp. 1-11, 2012.
T. Massoud, V. Maurice, L. H. Klein, and P. Marcus, “Nanoscale morphology and atomic structure of passive films on stainless steel,” Journal of The Electrochemical Society, vol. 160, pp. C232-C238, 2013.
V. S. Saji, and C.-W. Lee, “Molybdenum, Molybdenum oxides, and their electrochemistry,” ChemSusChem, vol. 5, pp. 1146-1161, 2012.
P. Marcus, V. Maurice, and H.-H. Strehblow, “Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure,” Corrosion Science, vol. 50, pp. 2698-2704, 2008.
B. Zhang, Y. Li, and F. Wang, “Electrochemical corrosion behaviour of microcrystalline aluminium in acidic solutions,” Corrosion Science, vol. 49, no. 5, pp. 2071-2082, 2007.
S. V. Muley, A. N. Vidvans, G. P. Chaudhari, and S. Udainiya, “An assessment of ultra-fine grained 316L stainless steel for implant applications,” Acta Biomaterialia, vol. 30, pp. 408-419, 2016.
M. Tingjiang, and X. Naixin, “The galvanostatic potential oscillations of mild steel in 93% sulfuric acid,” Corrosion Science, vol. 34, pp. 915-920, 1993.
W. Kuang, X. Wu, and E.-H. Han, “The oxidation behaviour of 304 stainless steel in oxygenated high temperature water,” Corrosion Science, vol. 52, pp. 4081-4087, 2010.
P. Keller, and H.-H. Strehblow, “XPS investigations of electro-chemically formed passive layers on Fe/Cr- alloys in 0.5 M H2SO4,” Corrosion Science, vol. 46, pp. 1939-1952, 2004.
M. Lodhi, K. Deen, and W. Haider, “Corrosion behavior of additively manufactured 316L stainless steel in acidic media,” Materialia, vol. 2, pp. 111-121, 2018.
L. Monaco, G. Avramovic-Cingara, G. Palumbo and ,U. Erb, “Corrosion behaviour of electrodeposited nanocrystalline nickel-iron (NiFe) alloys in dilute H2SO4,” Corrosion Science, vol. 130, pp. 103-112, 2018.
A. Pardo, M. C. Merino, M. Carboneras, F. Viejo, R. Arrabal, and J. Munoz, “Influence of Cu and Sn content in the corrosion of AISI 304 and 316 stainless steels in H2SO4,” Corrosion Science, vol. 8, pp. 1075-1092, 2006.
J. Ding, Z. Lei, L. Minxu, W. Jing, W. Zhibin, and H. Wenhui, “The electrochemical behaviour of 316L austenitic stainless steel in Cl− containing environment under different H2S partial pressures,” Applied Surface Science, vol. 289, pp. 33-41, 2014.
Q. Guo, J. Liu, M. Yu, and S. Li, “Effect of passive film on mechanical properties of martensitic stainless steel 15-5 pH in a neutral NaCl solution,” Applied Surface Science, vol. 327, pp. 313-320, 2015.
R. Sueptitz, K. Tschulik, M. Uhlemann, L. Schultz, and A. Gebert, “Effect of high gradient magnetic fields on the anodic behaviour and localized corrosion of iron in sulphuric acid solutions,” Corrosion Science, vol. 53, pp. 3222-3230, 2011.
R. T. Loto, C. A. Loto, A. P. I. Popoola, and M. Ranyaoa, “Corrosion resistance of austenitic stainless steel in sulphuric acid,” International journal of physical sciences, vol. 7, pp. 1677-1688, 2012.
H. Luo, C. Dong, K. Xiao, and X. Li, “The passive behaviour of ferritic stainless steel containing alloyed tin in acidic media,” The Royal Society of Chemistry, vol. 6, pp. 9940-9949, 2016.
Z. Wang, L. Zhang, X. Tang, Z. Zhang, and M. Lu, “The surface characterization and passive behavior of Type 316L stainless steel in H2S-containing conditions,” Applied Surface Science, vol. 423, pp. 457-464, 2017.
NACE, RP0391-2001: Standard Recommended Practice – Materials for the Handling and Storage of Commercial Concentrated (90 to 100%) Sulfuric Acid at Ambient Temperatures Houston: National Association of Corrosion Engineers. 2001.
Y. Li, M. Ives, K. Coley, and J. Rodda, “Corrosion of nickel-containing stainless steel in concentrated sulphuric acid,” Corrosion Science, vol. 46, pp. 1969-1979, 2004.
B. T. Ellison, and W. R. Schmeal, “Corrosion of steel in concentrated sulfuric acid,” Journal of The Electrochemical Society, vol. 125, pp. 524-531, 1978.
J. Hines, and R. Williamson, “Anodic behaviour of mild steel in strong sulphuric acid-I. Steady-state conditions,” Corrosion Science, vol. 4, pp. 201-210, 1964.
S. Jeon, H. Kim, K. Kong, and Y. Park, “Effects of copper addition on the passivity and corrosion behavior of 27Cr-7Ni hyper duplex stainless steels in sulfuric acid solution,” Materials transactions, vol. 56, pp. 78-84, 2015.
J. Wang, J. Wang, and E.-H. Han, “Influence of conductivity on corrosion behavior of 304 stainless steel in high temperature aqueous environment,” Journal of Materials Science & Technology, vol.32, pp. 333-340, 2016.
H. Sun, X. Wu, and E.-H. Han, “Effects of temperature on the oxide film properties of 304 stainless steel in high temperature lithium borate buffer solution,” Corrosion Science, vol. 51, pp. 2840-2847, 2009.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












