Arginine-modified surface coffee grounds activated carbon for Pb\(^{2+}\) adsorption: Kinetic, isotherm and thermodynamic studies
DOI:
https://doi.org/10.55713/jmmm.v35i1.2148Keywords:
Lead, Arginine, Adsorption, Desorption, Activated carbonAbstract
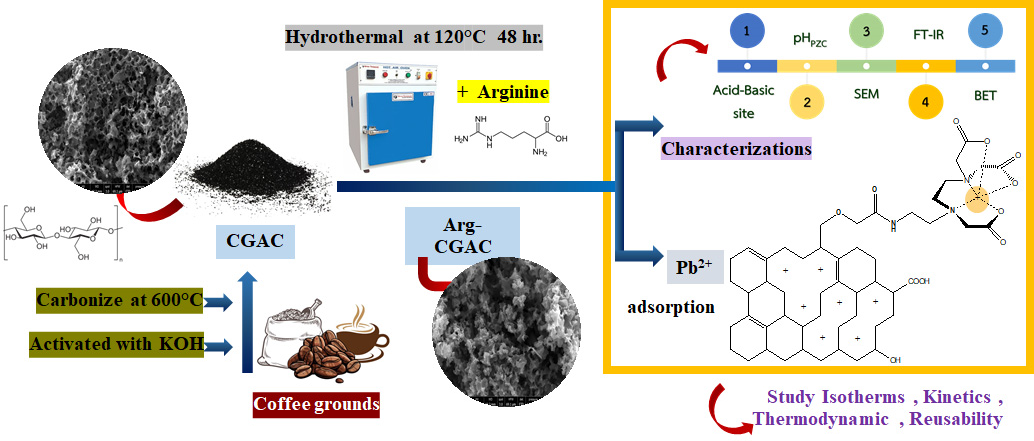
This research focuses on enhancing the value of coffee waste by converting it into activated carbon for removing Pb²⁺ ions from contaminated water. Coffee grounds were activated with KOH and carbonized at 600°C for 4 h to produce activated carbon (CGAC), then modified with arginine via hydrothermal treatment at 120°C for 48 h to create Arg-CGAC. The adsorbents' chemical and physical properties were analyzed using SEM, FT-IR, BET, and pHpzc methods. Batch experiments revealed that Arg-CGAC exhibited superior Pb2+ adsorption (223.10 mg∙g‒1) compared to CGAC (119.23 mg∙g‒1) under optimal conditions. Isotherm analysis showed stronger adsorbate-adsorbent interactions and a more endothermic adsorption for Arg-CGAC. Arg-CGAC demonstrated both physisorption and weak chemisorption, while CGAC primarily exhibited physisorption. Increasing temperatures made ΔG° values more negative, indicating more favorable adsorption for Arg-CGAC. Desorption tests showed a 98% yield over four cycles, but efficiency declined after the 7th cycle, stabilizing at 55% to 60%, suggesting good initial performance despite some regeneration limitations.
Downloads
References
O. Alam, and X. Qiao, "Influences of chemically controlled Ca-bearing minerals in chitosan on Pb2+ removal efficiency," Journal of Environmental Health Science and Engineering, vol. 18, no. 2, pp. 993-1005, 2020. DOI: https://doi.org/10.1007/s40201-020-00521-9
O. Alam, X. J. Zheng, D. Du, X. Qiao, L. Dai, J.. Li, J. Xia, J. Ye, and S. Zhong, "A critical review on advances in remediation of toxic heavy metals contaminated solids by chemical processes," Journal of Environmental Chemical Engineering, vol. 12, no. 4, p. 113149, 2024. DOI: https://doi.org/10.1016/j.jece.2024.113149
I. Ali, C. Peng, D. Lin, D. P. Saroj, I. Naz, Z. M. Khan, M. Sultan, and M. Ali, "Encapsulated green magnetic nanoparticles for the removal of toxic Pb2+ and Cd2+ from water: Development, characterization and application," Journal of Environmental Management, vol. 234, no. 2019, pp. 273-289, 2019. DOI: https://doi.org/10.1016/j.jenvman.2018.12.112
G. Murtaza, A. Ditta, N. Ullah, M. Usman, and Z. Ahmed, "Biochar for the management of nutrient impoverished and metal contaminated soils: preparation, applications, and prospects," Journal of Soil Science and Plant Nutrition, vol. 21, no. 3, pp. 2191-2213, 2021. DOI: https://doi.org/10.1007/s42729-021-00514-z
S. Bahah, "Analytical study on lead elimination by anionic clays: Characterization, adsorption kinetics, isotherm, thermo-dynamic, mechanism and adsorption," Analytical Methods in Environmental Chemistry Journal, vol. 6, no. 3, pp. 67-88, 2023. DOI: https://doi.org/10.24200/amecj.v6.i03.248
O. Uygun, A. Murat, and G. O. Çakal, "A novel magnetic sepiolite/ Fe2O3 composite for adsorptive removal of lead(II) ions from aqueous solutions," Clay Minerals, vol. 58, no. 3, pp. 1-32, 2023. DOI: https://doi.org/10.1180/clm.2023.24
A. El Mouden, N. El Messaoudi, A. El Guerrak, A. Bouich, V. Mehmeti, A. Lacherai, A. Jada, and J. H. P. Americo-Pinheiro, "Removal of cadmium and lead ions from aqueous solutions by novel dolomite-quartz@Fe3O4 nanocomposite fabricated as nanoadsorbent," Environmental Research, vol. 225, no. 2022, p. 115606, 2023. DOI: https://doi.org/10.1016/j.envres.2023.115606
C. Shao, F. Fan, and Y. Dai, "Lead ions removal from water by tartaric acid modified biochar materials: Equilibrium, kinetic studies and mechanism," Desalination and Water Treatment, vol. 320, p. 100601, 2024. DOI: https://doi.org/10.1016/j.dwt.2024.100601
P. Kongsune, S. Rattanapan, and R. Chanajaree, "The removal of Pb2+ from aqueous solution using mangosteen peel activated carbon: Isotherm, kinetic, thermodynamic and binding energy calculation," Groundwater for Sustainable Development, vol. 12, p. 100524, 2021. DOI: https://doi.org/10.1016/j.gsd.2020.100524
S. Rattanapan, J. Srikram, and P. Kongsune, "Adsorption of methyl orange on coffee grounds activated carbon," Energy Procedia, vol. 138, pp. 949-954, 2017. DOI: https://doi.org/10.1016/j.egypro.2017.10.064
S. Rattanaphan, T. Rungrotmongkol, and P. Kongsune, "Biogas improving by adsorption of CO2 on modified waste tea activated carbon," Renewable Energy, vol. 145, pp. 622-631, 2020. DOI: https://doi.org/10.1016/j.renene.2019.05.104
D. Khater, M. Alkhabbas, and A. M. Al-Ma’abreh, "Adsorption of Pb, Cu, and Ni ions on activated carbon prepared from oak cupules: Kinetics and thermodynamics studies," Molecules, vol. 29, no. 11, p. 2489, 2024. DOI: https://doi.org/10.3390/molecules29112489
M. S. Hossen, T. Islam, S. M. Hoque, A. Islam, M. M. Bashar, and G. Bhat, "Synthesis, activation, and characterization of carbon fiber precursor derived from jute fiber," ACS Omega, vol. 9, no. 33, pp. 35384-35393, 2024. DOI: https://doi.org/10.1021/acsomega.4c01268
M. C. Silva, L. H. S. Crespo, A. L. Cazetta, T. L. Silva, L. Spessato, and V. C. Almeida, "Activated carbon fibers of high surface area from corn husk: Mono and multicomponent adsorption studies of Pb2+ and Cu2+ ions from aqueous solution," Journal of Molecular Liquids, vol. 405, no. 2023, p. 124919, 2024. DOI: https://doi.org/10.1016/j.molliq.2024.124919
R. Campbell, B. Xiao, and C. Mangwandi, "Production of activated carbon from spent coffee grounds (SCG) for removal of hexavalent chromium from synthetic wastewater solutions," Journal of Environmental Management, vol. 366, p. 121682, 2024. DOI: https://doi.org/10.1016/j.jenvman.2024.121682
V. E. Pakade, L. M. Madikizela, M. J. Klink, and S. Ncube, "Adsorption of toxic heavy metals using charred and uncharred coffee waste adsorbents: a review," Environmental Technology Reviews, vol. 12, no. 1, pp. 359-389, 2023. DOI: https://doi.org/10.1080/21622515.2023.2215459
J. Chen, Y. Liu, J. Liu, Q. Duan, Z. Wang, J. Song, C. Ji, and J. Sun, "Effects of carbonization on the structure and sorption properties of coffee grounds for Pb(II) and Ni(II) in various metal systems," Desalination and Water Treatment, vol. 320, no. 9, p. 100623, 2024. DOI: https://doi.org/10.1016/j.dwt.2024.100623
X. Yang, Y. Wan, Y. Zheng, F. He, Z. Yu, J. Huang, H. Wang, Y. S. Ok, Y. Jiang, and B. Gao, "Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy
metals from aqueous solutions: A critical review," (in eng), Chemical Engineering Journal, vol. 366, pp. 608-621, 2019. DOI: https://doi.org/10.1016/j.cej.2019.02.119
B. Liu, P. Lv, Q. Wang, Y. Bai, J. Wang, W. Su, X. Song, and G. Yu, "Statistical physics investigation on the simultaneous adsorption mechanism of Cd2+ and Pb2+ on amino functionalized activated carbon derived from coal gasification fine slag," Journal of Environmental Chemical Engineering, vol. 12, no. 3, p. 112635. 2024. DOI: https://doi.org/10.1016/j.jece.2024.112635
M. Naushad, A. A. Alqadami, Z. A. AlOthman, I. H. Alsohaimi, M. S. Algamdi, and A. M. Aldawsari, "Adsorption kinetics, isotherm and reusability studies for the removal of cationic dye from aqueous medium using arginine modified activated carbon," Journal of Molecular Liquids, vol. 293, p. 111442, 2019. DOI: https://doi.org/10.1016/j.molliq.2019.111442
C. Zhang, S. Qi, J. Meng, and X. Chen, "Selective and efficient extraction of heparin by arginine-functionalized flowered meso-porous silica nanoparticles with high capacity," Separation and Purification Technology, vol. 276, p. 119321, 2021. DOI: https://doi.org/10.1016/j.seppur.2021.119321
W. Liu, J. Zhang, Y. Jin, X. Zhao, and Z. Cai, "Adsorption of Pb(II), Cd(II) and Zn(II) by extracellular polymeric substances extracted from aerobic granular sludge: Efficiency of protein," Journal of Environmental Chemical Engineering, vol. 3, no. 2, pp. 1223-1232, 2015. DOI: https://doi.org/10.1016/j.jece.2015.04.009
L. Brini, A. Hsini, Y. Naciri, A. Bouziani, Z. Ajmal, K. H' Maida, A. Boulahya, M. Arahou, B. Bakiz, A. Albourine, and F. Mohamed, "Synthesis and characterization of arginine-doped heliotrope leaves with high clean-up capacity for crystal violet dye from aqueous media," Water Science and Technology, vol. 84, no. 9, pp. 2265-2277, 2021. DOI: https://doi.org/10.2166/wst.2021.446
S. Brunauer, P. H. Emmett, and E. Teller, "Adsorption of gases in multimolecular layers," Journal of The American Chemical Society, vol. 60, no. 2, pp. 309-319, 1938. DOI: https://doi.org/10.1021/ja01269a023
N. Nikolić, V. Maksimovic, and A. Ljiljana, "Correlation of morphology and crystal structure of metal powders produced by electrolysis processes," Metals, vol. 11, p. 859, 2021. DOI: https://doi.org/10.3390/met11060859
I. Langmuir, "The adsorption of gases on plane surfaces of glass, mica, and platinum," Journal of the American Chemical Society, vol. 40, pp. 1361-1403, 1918. DOI: https://doi.org/10.1021/ja02242a004
H. M. F. Freundlich, "Over the Adsorption in Solution," The Journal of Physical Chemistry, vol. 57, pp. 385-470, 1906. DOI: https://doi.org/10.1515/zpch-1907-5723
M. J. Temkin, and V. Pyzhev, "Recent modifications to langmuir isotherms," Acta Physiochim U.R.S.S, vol. 12, pp. 217-225, 1940.
M. M. Dubinin, and L. V. Radushkevich, "The equation of the
characteristic curve of the activated charcoal," Proceedings of the Academy of Sciences of the USSR. Physical chemistry section, vol. 55, pp. 331-337, 1947.
Y. Chu, M. A. Khan, F. Wang, M. Xia, W. Lei, and S. Zhu, "Kinetics and equilibrium isotherms of adsorption of Pb(II) and Cu(II) onto raw and arginine-modified montmorillonite," Advanced Powder Technology, vol. 30, no. 5, pp. 1067-1078, 2019. DOI: https://doi.org/10.1016/j.apt.2019.03.002
W. Somyanonthanakun, R. Ahmed, V. Krongtong, and S. Thongmee, "Studies on the adsorption of Pb(II) from aqueous solutions using sugarcane bagasse-based modified activated carbon with nitric acid: Kinetic, isotherm and desorption," Chemical Physics Impact, vol. 6, p. 100181, 2023. DOI: https://doi.org/10.1016/j.chphi.2023.100181
A. Rehman, A. Naeem, I. Ahmad, D. Fozia, B. Almutairi, M. Aslam, M. Israr, B. O. Almutairi, and Z. Ullah, "Synthesis of plant-mediated iron oxide nanoparticles and optimization of chemically modified activated carbon adsorbents for removal of As, Pb, and Cd ions from wastewater," ACS Omega, vol. 9, no. 1, pp. 317-329, 2024. DOI: https://doi.org/10.1021/acsomega.3c05299
A. Arul, S. Kavitha, A. Anand Babu Christus, V. J. Surya, A. Ravikumar, and Y. Sivalingam, "Enhanced removal of Pb (II) and Cd (II) ions from aqueous systems using coated magnetic nanoparticles in activated carbon derived from corncob waste," Surfaces and Interfaces, vol. 40, p. 103095, 2023. DOI: https://doi.org/10.1016/j.surfin.2023.103095
S. M. Waly, A. M. El-Wakil, W. M. A. El-Maaty, and F. S. Awad, "Efficient removal of Pb(II) and Hg(II) ions from aqueous solution by amine and thiol modified activated carbon," Journal of Saudi Chemical Society, vol. 25, no. 8, p. 101296, 2021. DOI: https://doi.org/10.1016/j.jscs.2021.101296
L. Zhu, Y. Yao, D. Chen, and P. Lan, "The effective removal of Pb2+ by activated carbon fibers modified by l-cysteine: exploration of kinetics, thermodynamics and mechanism," RSC Advances, vol. 12, no. 31, pp. 20062-20073, 2022. DOI: https://doi.org/10.1039/D2RA01521H
M. S. Alhumaimess, "Sulfhydryl functionalized activated carbon
for Pb(II) ions removal: kinetics, isotherms, and mechanism," Separation Science and Technology, vol. 55, no. 7, pp. 1303-1316, 2020. DOI: https://doi.org/10.1080/01496395.2019.1589513
J. Kończyk, S. Żarska, and W. Ciesielski, "Adsorptive removal of Pb(II) ions from aqueous solutions by multi-walled carbon nanotubes functionalised by selenophosphoryl groups: Kinetic, mechanism, and thermodynamic studies," Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 575, pp. 271-282, 2019. DOI: https://doi.org/10.1016/j.colsurfa.2019.04.058
H. M. Alraddadi, T. M. Fagieh, E. M. Bakhsh, K. Akhtar, S. B. Khan, S. A. Khan, E. A. Bahaidarah, and T. A. Homdi, "Adsorptive removal of heavy metals and organic dyes by sodium alginate/coffee waste composite hydrogel," International Journal of Biological Macromolecules, vol. 247, p. 125708, 2023. DOI: https://doi.org/10.1016/j.ijbiomac.2023.125708
Downloads
Published
How to Cite
License
Copyright (c) 2025 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












