Magnetic nanoparticles coated with zwitterionic copolymer as an advanced material for rapid and instrument-free biomolecular detection in human serum
DOI:
https://doi.org/10.55713/jmmm.v32i4.1572Keywords:
Magnetite nanoparticles, Zwitterionic copolymer, Medical diagnostics, Agglutination assayAbstract
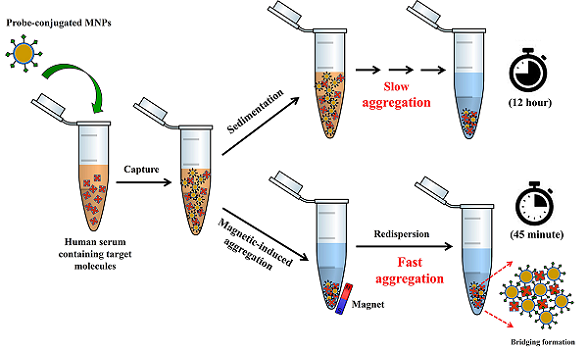
The traditional agglutination assays especially those based on polystyrene beads have been recognized as convenient tools for disease diagnosis despite their limited detection range and low sensitivity. Unlike other particles namely polystyrene beads, SiO2 and gold nanoparticles having insignificant magnetic properties, magnetic nanoparticles (MNPs) offer unique advantages as their magnetic properties for agglutination methods. In the presence of magnet, not only can they be used to enrich the samples, but also their aggregation can also be induced, providing sensitive and rapid measurements. This work aims to develop MNPs for aggregation-based biomolecular detection. The MNPs were surface-modified with PMAMPC via an in situ coating method, then biotin as the target-specific probe was immobilized. The biotin-conjugated PMAMPC-MNPs were used for capturing and detecting the complementary protein, streptavidin in human serum samples. With the magnetic-induction, the nanoparticles would aggregate in the presence of streptavidin, resulting in a short detection time even in undiluted human serum. The concentration range for the detection was 35 nM to 150 nM and the lowest concentration of detection was 35 nM or equivalent to 2.5 mg⸳mL-1. The fact that this is simple, rapid and instrument-free method for biomolecular detection broadens their potential use in a variety of diagnostic applications.
Downloads
References
D. R Brenner, D. Scherer, K. Muir, J. Schildkraut, P. Boffetta, M. R Spitz, L. L. Marchand , A. T. Chan, E. L Goode, C. M Ulrich, and R. J. Hung "A review of the application
of inflammatory biomarkers in epidemiologic cancer research," Cancer Epidemiology, Biomarkers & Prevention, vol. 23, no. 9, pp. 1729-1751, 2014.
S.-E. Kim, Y. J. Kim, S. Song, K.-N. Lee, and W. K. Seong, "A simple electrochemical immunosensor platform for detection of apolipoprotein A1 (Apo-A1) as a bladder cancer biomarker in urine," Sensors and Actuators B: Chemical, vol. 278, pp. 103-109, 2019.
K. Noi, A. Iwata, F. Kato, and H. Ogi, "Ultrahigh-frequency, wireless MEMS QCM biosensor for direct, label-free detection of biomarkers in a large amount of contaminants," Analytical chemistry, vol. 91, no. 15, pp. 9398-9402, 2019.
B. Yuan, S. Schafferer, Q. Tang, M. Scheffler, J. Nees, J. Heil, S. Schott, M. Golatta, M Wallwiener, C. Sohn, T. Koal, B. Wolf, A. Schneeweiß, B. Burwinkel"A plasma metabolite panel as biomarkers for early primary breast cancer detection," International journal of cancer, vol. 144, no. 11, pp. 2833-2842, 2019.
S.-H. Kwak, J.-S. Wi, J. Lee, C. Kim, and H.-K. Na, "Enhanced detection sensitivity through enzyme-induced precipitate accumulation in LSPR-active nano-valleys," RSC advances, vol. 12, no. 25, pp. 15652-15657, 2022.
A. Uniyal, A. Pal, and Vivek Srivastava, "Advances in surface plasmon resonance–based biosensor technologies for cancer biomarker detection," Biosensors and Bioelectronics, vol. 197, p. 113767, 2022.
S. Esmail, M. J. Knauer, H. Abdoh, C. Voss, B. Chin-Yee, P. Stogios, A. Seitova, A. Hutchinson, F. Yusifov, T. Skarina, E. Evdokimova, S. Ackloo, L. Lowes, B. D. Hedley, V. Bhayana, I. Chin-Yee and S. S-C. Li, "Rapid and accurate agglutination-based testing for SARS-CoV-2 antibodies," Cell reports methods, vol. 1, no. 2, p. 100011, 2021.
J. Li, A. Concellón, K. Yoshinaga, Z. Nelson, Q. He, and T. M. Swager, "Janus emulsion biosensors for anti-SARS-CoV-2 spike antibody," ACS Central Science, vol. 7, no. 7, pp. 1166-1175, 2021.
V. Kesarwani, J. A. Walker, E. C. Henderson, G. Huynh, H. McLiesh, M. Graham, M. Wieringa, M. M. B. Holl, G. Garnier, and S. R. Corrie, "Column agglutination assay using polystyrene microbeads for rapid detection of antibodies against SARS-CoV-2," ACS Applied Materials & Interfaces, vol. 14, no. 2, pp. 2501-2509, 2022.
K. Sato, K. Hosokawa, and M. Maeda, "Rapid aggregation of gold nanoparticles induced by non-cross-linking DNA hybridization," Journal of the American Chemical Society, vol. 125, no. 27, pp. 8102-8103, 2003.
Y. Iwasaki, T. Kimura, M. Orisaka, H. Kawasaki, T. Goda, and S.-i. Yusa, "Label-free detection of C-reactive protein using highly dispersible gold nanoparticles synthesized by reducible biomimetic block copolymers," Chemical Communications, vol. 50, no. 42, pp. 5656-5658, 2014.
Y.-S. Borghei, M. Hosseini, M. Dadmehr, S. Hosseinkhani, M. R. Ganjali, and R. Sheikhnejad, "Visual detection of cancer cells by colorimetric aptasensor based on aggregation of gold nanoparticles induced by DNA hybridization," Analytica chimica acta, vol. 904, pp. 92-97, 2016.
S. Iwasaki, H. Kawasaki, and Y. Iwasaki, "Label-free specific detection and collection of c-reactive protein using zwitterionic phosphorylcholine-polymer-protected magnetic nanoparticles," Langmuir, vol. 35, no. 5, pp. 1749-1755, 2018.
E. Pinchon, F. Leon, N. Temurok, F. Morvan, J-J. Vasseur, M. Clot, V. Foulongne, J-F. Cantaloube, P. V. Perre, A. Daynès, J-P, Molès, and C. Fournier-Wirth, "Rapid and specific DNA detection by magnetic field-enhanced agglutination assay," Talanta, vol. 219, p. 121344, 2020.
F. Leon, E. Pinchon, N. Temurok, F. Morvan, J-J Vasseur, M. Clot, V. Foulongne,1 J-F. Cantaloube, P. V. Perre, J-P Molès, A. Daynès, and C. Fournier-Wirth, "Diagnostic performance of a magnetic field-enhanced agglutination readout in detecting either viral genomes or host antibodies in arbovirus infection," Microorganisms, vol. 9, no. 4, p. 674, 2021.
F. Leon, E. Pinchon, C. Mayran, A. Daynès, F Morvan, J-P Molès, J-F Cantaloube, and C. Fournier-Wirth, "Magnetic field-enhanced agglutination readout combined with isothermal reverse transcription recombinase polymerase amplification for rapid and sensitive molecular detection of dengue virus," Frontiers in chemistry, vol. 9, p. 817246, 2021.
Y. Mao, X. Huang, S. Xiong, H. Xu, Z. P. Aguilar, and Y. Xiong, "Large-volume immunomagnetic separation combined with multiplex PCR assay for simultaneous detection of Listeria monocytogenes and Listeria ivanovii in lettuce," Food Control, vol. 59, pp. 601-608, 2016.
Y. Wan, Y. Sun, P. Qi, P. Wang, and D. Zhang, "Quaternized magnetic nanoparticles–fluorescent polymer system for detection and identification of bacteria," Biosensors and Bioelectronics, vol. 55, pp. 289-293, 2014.
T. Xue, S. Wang, G. Ou, Y. Li, H. Ruan, Z. Li, Y. Ma, R. Zou, J. Qiu, Z. Shen, and A. Wu, "Detection of circulating tumor cells based on improved SERS-active magnetic nanoparticles," Analytical Methods, vol. 11, no. 22, pp. 2918-2928, 2019.
R-S. Juang, W-T. Chen, Y-W. Cheng, K-S. Wang, R-J. Jeng, Z-L. Zeng, S-H. Liu, and T-Y. Liu, "Fabrication of in situ magnetic capturing and Raman enhancing nanoplatelets for detection of bacteria and biomolecules," Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 648, p. 129189, 2022.
Z. Zhang, Z. Wang, X. Wang, and X. Yang, "Magnetic nanoparticle-linked colorimetric aptasensor for the detection of thrombin," Sensors and Actuators B: Chemical, vol. 147, no. 2, pp. 428-433, 2010.
Y. Xianyu, Y. Dong, Z. Wang, Z. Xu, R. Huang, and Y. Chen, "Broad-range magnetic relaxation switching bioassays using click chemistry-mediated assembly of polystyrene beads and magnetic nanoparticles," ACS sensors, vol. 4, no. 7, pp. 1942-1949, 2019.
H. Zhang, C. Fu, S. Wu, Y. Shen, C. Zhou, J. Neng, Y. Yi, Y. Jin, and Y. Zhu, "Magnetic-capture-based SERS detection of multiple serum microRNA biomarkers for cancer diagnosis," Analytical Methods, vol. 11, no. 6, pp. 783-793, 2019.
I.-H. Cho and J. Irudayaraj, "In-situ immuno-gold nanoparticle network ELISA biosensors for pathogen detection," International journal of food microbiology, vol. 164, no. 1, pp. 70-75, 2013.
G. Bayramoglu, V. C. Ozalp, M. Oztekin, and M. Y. Arica, "Rapid and label-free detection of Brucella melitensis in milk and milk products using an aptasensor," Talanta, vol. 200, pp. 263-271, 2019.
K. G. Neoh, and E. T. Kang, "Functionalization of inorganic nanoparticles with polymers for stealth biomedical applications," Polymer Chemistry, vol. 2, no. 4, pp. 747-759, 2011.
S. Boonjamnian, T. Trakulsujaritchok, K. Srisook, V. P. Hoven, and P. N. Nongkhai, "Biocompatible zwitterionic copolymer-stabilized magnetite nanoparticles: a simple one-pot synthesis, antifouling properties and biomagnetic separation," RSC advances, vol. 8, no. 65, pp. 37077-37084, 2018.
P. Akkahat, S. Kiatkamjornwong, S.-i. Yusa, V. P. Hoven, and Y. Iwasaki, "Development of a novel antifouling platform for biosensing probe immobilization from methacryloyloxyethyl phosphorylcholine-containing copolymer brushes," Langmuir, vol. 28, no. 13, pp. 5872-5881, 2012.
E. A Gould, A. Buckley, and N. Cammack. "Use of the biotin-streptavidin interaction to improve flavivirus detection by immunofluorescence and ELISA tests," Journal of virological methods, no. 1, pp. 41-48, 1985.
M. Zhu, G. Xue, H. Yonghong, O. Weijun, and W. Yakun. "Streptavidin-biotin-based directional double Nanobody sandwich ELISA for clinical rapid and sensitive detection of influenza H5N1," Journal of translational medicine, vol. 12, no. 1, pp. 1-10, 2014.
M. De, O. R. Miranda, S. Rana, and V. M. Rotello, "Size and geometry dependent protein–nanoparticle self-assembly," Chemical communications, no. 16, pp. 2157-2159, 2009.
A. Blanco, and G. Blanco, Medical biochemistry. Academic Press, 2017.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












