Corrosion of a spark plasma sintered Fe-Cr-Mo-B-C alloy in 3.5% NaCl solution
DOI:
https://doi.org/10.55713/jmmm.v33i1.1588คำสำคัญ:
Corrosion, Powder Metallurgy, Amorphous Alloy Powder, Passive Filmบทคัดย่อ
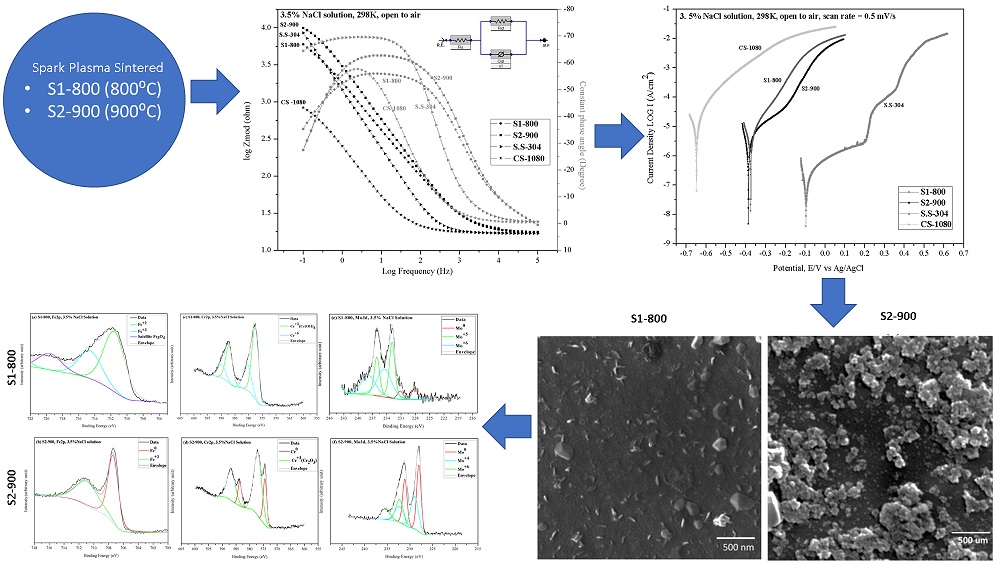
In this study, the corrosion behavior of a Fe-Cr-Mo-B-C alloy, fabricated by spark plasma sintering of an amorphous alloy powder, in 3.5% NaCl solution was analyzed. Electrochemical impedance spectroscopy and potentiodynamic polarization are techniques which were used for electrochemical performance estimation of samples and the results were further compared with conventional alloys: 1080 carbon steel and 304 stainless steel. Corrosion surface products were characterized through Scanning electron microscopy, Energy dispersive x-ray spectroscopy and X-ray photoelectron spectroscopy. Specimens sintered at 800℃ (S1-800) had achieved 94% densification approximately while the sample sintered at 900℃ (S2-900), had densified more which was 98% approximately. S2-900 had better corrosion resistance than S1-800 while in comparison to conventional alloys; it was inferior to 304 stainless steel. It was concluded that the increase in density of sintered samples favoured the formation of more uniform surface products and enhanced the formation of the passive chromium oxide (Cr2O3) layer.
Downloads
เอกสารอ้างอิง
S. Pang, T. Zhang, K. Asami, and A. Inoue, “Effects of chromium on the glass formation and corrosion behavior of bulk glassy Fe-Cr-Mo-C-B Alloys.,” Materials Transactions, vol. 43, no. 8, pp. 2137-2142, 2002.
Z. M. Wang, Y. T. Ma, J. Zhang, W. L. Hou, X. C. Chang, and J. Q. Wang, “Influence of yttrium as a minority alloying element on the corrosion behavior in Fe-based bulk metallic glasses,” Electrochimica Acta, vol. 54, no. 2, pp. 261-269, 2008.
M. S. Bakare, K. T. Voisey, K. Chokethawai, and D. G. McCartney, “Corrosion behaviour of crystalline and amorphous forms of the glass forming alloy Fe43Cr16Mo16C15B10,” Journal of Alloys and Compounds, vol. 527, pp. 210-218, 2012.
J. Jayaraj, Y. C. Kim, K. B. Kim, H. K. Seok, and E. Fleury, “Corrosion behaviors of Fe45-xCr18Mo14C15B6Y2Mx (M = Al, Co, Ni, N and x = 0, 2) bulk metallic glasses under conditions simulating fuel cell environment,” Journal of Alloys and Compounds, vol. 434-435, no. SPEC. ISS., pp. 237-239, 2007.
O. A. Graeve, R. Kanakala, L. Kaufman, K. Sinha, E.Wang, B. Pearson, G. Rojas-George, J. C. Farmer, “Spark plasma sintering of Fe-based structural amorphous metals (SAM) with Y2O3 nanoparticle additions,” Materials Letters, vol. 62, no. 17-18, pp. 2988-2991, 2008.
S. L. Wang, H. X. Li, X. F. Zhang, and S. Yi, “Effects of Cr contents in Fe-based bulk metallic glasses on the glass forming ability and the corrosion resistance,” Materials Chemistry and Physics, vol. 113, no. 2-3, pp. 878-883, 2009.
J. Eckert, J. Das, S. Pauly, and C. Duhamel, “Mechanical properties of bulk metallic glasses and composites,” Journal of Materials Research, vol. 22, no. 02, pp. 285-301, 2007.
S. Madge, “Toughness of bulk metallic glasses,” Metals, vol. 5, no. 3, pp. 1279-1305, 2015.
C. Suryanarayana, and A. Inoue, “Iron-based bulk metallic glasses,” International Materials Reviews, vol. 58, no. 3, pp. 131-166, 2013.
M. M. Trexler, and N. N. Thadhani, “Mechanical properties of bulk metallic glasses,” Progress in Materials Science, vol. 55, no. 8, pp. 759-839, 2010.
J. Jayaraj, K. B. Kim, H. S. Ahn, and E. Fleury, “Corrosion mechanism of N-containing Fe-Cr-Mo-Y-C-B bulk amorphous alloys in highly concentrated HCl solution,” Materials Science and Engineering A, vol. 449-451, pp. 517-520, 2007.
N. Chen, L. Martin, D. V. Luzguine-Luzgin, and A. Inoue, “Role of alloying additions in glass formation and properties of bulk metallic glasses,” Materials, vol. 3, no. 12, pp. 5320-5339, 2010.
A. Inoue, B. Shen, and A. Takeuchi, “Developments and applications of bulk glassy alloys in late transition metal base system,” Materials Transactions, vol. 47, no. 5, pp. 1275-1285, 2006.
J. Schroers, “Processing of bulk metallic glass,” Advanced Materials, vol. 22, no. 14, pp. 1566-1597, 2010.
S. F. Guo, K. C. Chan, S. H. Xie, P. Yu, Y. J. Huang, and H. J. Zhang, “Novel centimeter-sized Fe-based bulk metallic glass with high corrosion resistance in simulated acid rain and seawater,” Journal of Non-Crystalline Solids, vol. 369, pp. 29-33, 2013.
G. Kumar, A. Desai, and J. Schroers, “Bulk metallic glass: The smaller the better,” Advanced Materials, vol. 23, no. 4, pp. 461-476, 2011.
M. Park, American Society for Metals Handbook Corrosion : Materials, vol. 13. 2005.
M. Ramya, S. G. Sarwat, V. Udhayabanu, S. Subramanian, B. Raj, and K. R. Ravi, “Role of partially amorphous structure and alloying elements on the corrosion behavior of Mg-Zn-Ca bulk metallic glass for biomedical applications,” Materials and Design, vol. 86, pp. 829-835, 2015.
A. Concustell, G. Alcala, S. Mato, T.G. Woodcock, A. Gebert, J. Eckert, and M.D. Baro, “Effect of relaxation and primary nanocrystallization on the mechanical properties of Cu60Zr22Ti18 bulk metallic glass,” Intermetallics, vol. 13, no. 11, pp. 1214-1219, 2005.
K. Mondal, T. Ohkubo, T. Toyama, Y. Nagai, M. Hasegawa, and K. Hono, “The effect of nanocrystallization and free volume on the room temperature plasticity of Zr-based bulk metallic glasses,” Acta Materialia, vol. 56, no. 18, pp. 5329-5339, 2008.
J. Fornell, E. Rossinyol, S. Suriñach, M. D. Baró, W. H. Li, and J. Sort, “Enhanced mechanical properties in a Zr-based metallic glass caused by deformation-induced nanocrystallization,” Scripta Materialia, vol. 62, no. 1, pp. 13-16, 2010.
M. Chen, A. Inoue, W. Zhang, and T. Sakurai, “Extraordinary plasticity of ductile bulk metallic glasses,” Physical Review Letters, vol. 96, no. 24, 2006.
X. Li, H. Kato, K. Yubuta, A. Makino, and A. Inoue, “Improved plasticity of iron-based high-strength bulk metallic glasses by copper-induced nanocrystallization,” Journal of Non-Crystalline Solids, vol. 357, no. 15, pp. 3002-3005, 2011.
H. Y. Jung, and S. Yi, “Nanocrystallization and soft magnetic properties of Fe23M6 (M: C or B) phase in Fe-based bulk metallic glass,” Intermetallics, vol. 49, pp. 18-22, 2014.
L. Liu, Y. Li, and F. Wang, “Electrochemical corrosion behavior of nanocrystalline materials—a Review,” Journal of Materials Science and Technology, vol. 26, no. 1, pp. 1-14, 2010.
C. A. C. Souza, D. V Ribeiro, and C. S. Kiminami, “Corrosion resistance of Fe-Cr-based amorphous alloys : An overview,” Journal of Non-Crystalline Solids, vol. 442, pp. 56-66, 2016.
L. Wang, J. Zhang, and W. Jiang, “Recent development in reactive synthesis of nanostructured bulk materials by spark plasma sintering,” International Journal of Refractory Metals and Hard Materials, vol. 39, pp. 103-112, 2013.
F. Watari, A.Yokoyama, M. Omori, T. Hirai, H. Kondo, M. Uo, and T. Kawasaki, “Biocompatibility of materials and development to functionally graded implant for bio-medical application,” Composites Science and Technology, vol. 64, no. 6, pp. 893-908, 2004.
A. A. Sorour, M. Farooq, A. Mekki, and A. M. Kumar, “Corrosion of a spark plasma sintered Fe ‑ Cr ‑ Mo ‑ B ‑ C alloy in hydrochloric acid,” Metallography, Microstructure, and Analysis, vol. 10, no. 3, pp. 291-301, 2021.
D. Wallinder, J. Pan, C. Leygraf, and A. Delblanc-Bauer, “EIS and XPS study of surface modification of 316LVM stainless steel after passivation,” Corrosion Science, vol. 41, no. 2, pp. 275-289, 1998.
R. Solmaz, G. Kardaş, M. Çulha, B. Yazici, and M. Erbil, “Investigation of adsorption and inhibitive effect of 2-mercaptothiazoline on corrosion of mild steel in hydrochloric acid media,” Electrochimica Acta, vol. 53, no. 20, pp. 5941-5952, 2008.
E. McCafferty, Introduction to Corrosion Science, First. Springer, 2009.
M. Vayer, I. Reynaud, and R. Erre, “XPS characterisations of passive films formed on martensitic stainless steel: qualitative and quantitative investigations,” Journal of Materials Science, vol. 35, no. 10, pp. 2581-2587, 2000.
S. D. Zhang, J. Wu, W. B. Qi, and J. Q. Wang, “Effect of porosity defects on the long-term corrosion behaviour of Fe-based amorphous alloy coated mild steel,” Corrosion Science, vol. 110, pp. 57-70, 2016.
D. S. Petrovič, and D. Mandrino, “XPS characterization of the oxide scale on fully processed non-oriented electrical steel sheet,” Materials Characterization, vol. 62, no. 5, pp. 503-508, 2011.
T. Yamashita, and P. Hayes, “Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials,” Applied Surface Science, vol. 254, no. 8, pp. 2441-2449, 2008.
X. Y. Li, E. Akiyama, H. Habazaki, A. Kawashima, K. Asami, and K. Hashimoto, “Electrochemical and XPS studies of the corrosion behavior of sputter-deposited amorphous Fe-Cr-Ni-Nb alloys in 6 M HCl,” Corrosion Science, vol. 41, no. 6, pp. 1095-1118, 1999.
M.-D. N., P. M. A. Galtayries, and R. Warocquier-Cle´ rout, “Fibronectin adsorption on Fe–Cr alloy studied by XPS,” Surface and Interface Analysis, vol. 38, pp. 186-190, 2006.
C. O. A. Olsson, H. J. Mathieu, and D. Landolt, “Angle-resolved XPS analysis of molybdenum and tungsten in passive films on stainless steel PVD alloys,” Surface and Interface Analysis, vol. 34, no. 1, pp. 130-134, 2002.
ดาวน์โหลด
เผยแพร่แล้ว
วิธีการอ้างอิง
ฉบับ
บท
การอนุญาต
ลิขสิทธิ์ (c) 2023 วารสารโลหะ, วัสดุ และแร่

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












