pH-responsive polymeric nanostructures for cancer theranostics
DOI:
https://doi.org/10.55713/jmmm.v33i2.1609คำสำคัญ:
pH-responsive polymer, pH-triggered drug release, cancer theranostics, pH-sensitive nanocarriers, modification strategyบทคัดย่อ
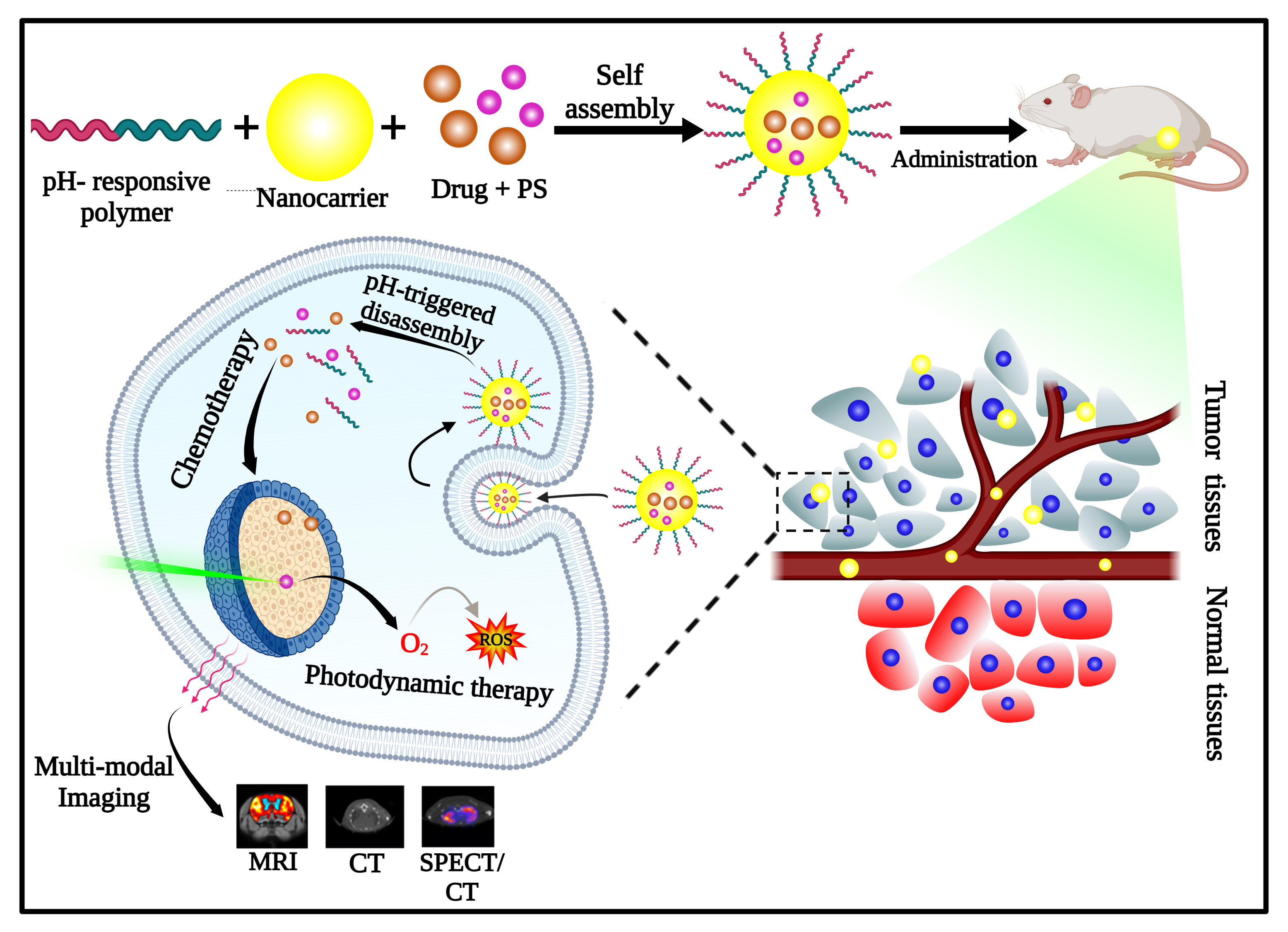
Responsive polymeric nanostructures are being designed to improve the efficiency of existing treatment techniques by delivering therapeutics in precise locations. The properties of the particles can be altered to act as a probe for imaging applications also. Hence, an effective theranostic agent can be tailor-made to meet the requirements. The pH variability has aroused considerable interest in nano-responsive-stimulus production since the mild acidic condition is a hallmark of the tumor microenvironment. The cargo sealed inside the carrier will be released either by swelling or disassembly of the carrier as they meet a pH drop. The modification strategy for the synthesis of pH-responsive polymers is discussed in the manuscript. Fabrication of pH-responsive theranostic agents can conquer major limitations of conventional treatment techniques. Herein we reported imperative insights on recent pH-sensitive polymeric nanomaterials for the treatment of various disease conditions, especially cancer.
Downloads
เอกสารอ้างอิง
C. Gao, F. Tang, G. Gong, J. Zhang, M. P. Hoi, S. M. Lee, and R. Wang, "pH-responsive prodrug nanoparticles based on a sodium alginate derivative for selective co-release of doxorubicin and curcumin into tumor cells," Nanoscale, vol. 9, no. 34, pp. 12533-12542, 2017.
A. Girigoswami, W. Yassine, P. Sharmiladevi, V. Haribabu, and K. Girigoswami, "Camouflaged nanosilver with excitation wavelength dependent high quantum yield for targeted theranostic," Scientific reports, vol. 8, no. 1, pp. 1-7, 2018.
S. W. Vedakumari, R. Senthil, S. Sekar, C. S. Babu, and T. P. Sastry, "Enhancing anti-cancer activity of erlotinib by antibody conjugated nanofibrin-in vitro studies on lung adenocarcinoma cell lines," Materials Chemistry and Physics, vol. 224, pp. 328-333, 2019.
J. K. Patra, G. Das, L. F. Fraceto, E. V. R. Campos, M. d. P. Rodriguez-Torres, L. S. Acosta-Torres, L. A. Diaz-Torres, R. Grillo, M. K. Swamy, and S. Sharma, "Nano based drug delivery systems: recent developments and future prospects," Journal of nanobiotechnology, vol. 16, no. 1, pp. 1-33, 2018.
A. López Ruiz, A. Ramirez, and K. McEnnis, "Single and multiple stimuli-responsive polymer particles for controlled drug delivery," Pharmaceutics, vol. 14, no. 2, p. 421, 2022.
S. S. Das, P. Bharadwaj, M. Bilal, M. Barani, A. Rahdar, P. Taboada, S. Bungau, and G. Z. Kyzas,"Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis," Polymers, vol. 12, no. 6, p. 1397, 2020.
S. De, S. Das, and A. Girigoswami, "Spectroscopic probing of bile salt–albumin interaction," Colloids and Surfaces B: Biointerfaces, vol. 54, no. 1, pp. 74-81, 2007.
S. Mosleh-Shirazi, M. Abbasi, A. Vaez, M. Shafiee, S.R. Kasaee, A. M. Amani, and S. Hatam, "Nanotechnology Advances in the detection and treatment of cancer: An overview," Nano-theranostics, vol. 6, no. 4, pp. 400-423, 2022.
S. De, A. Gopikrishna, V. Keerthana, A. Girigoswami, and K. Girigoswami, "An overview of nanoformulated nutraceuticals and their therapeutic approaches," Current Nutrition & Food Science, vol. 17, no. 4, pp. 392-407, 2021.
K. Harini, P. Pallavi, P. Gowtham, K. Girigoswami, and A. Girigoswami, "Smart polymer-based reduction responsive therapeutic delivery to cancer cells," Current Pharmacology Reports, pp. 1-7, 2022.
P. Sharmiladevi, N. Akhtar, V. Haribabu, K. Girigoswami, S. Chattopadhyay, and A. Girigoswami, "Excitation wavelength independent carbon-decorated ferrite nanodots for multimodal diagnosis and stimuli responsive therapy," ACS Applied Bio Materials, vol. 2, no. 4, pp. 1634-1642, 2019.
P. Sharmiladevi, M. Breghatha, K. Dhanavardhini, R. Priya, K. Girigoswami, and A. Girigoswami, "Efficient wormlike micelles for the controlled delivery of anticancer drugs," Nanoscience & Nanotechnology-Asia, vol. 11, no. 3, pp. 350-356, 2021.
N. Deirram, C. Zhang, S. S. Kermaniyan, A. P. Johnston, and G. K. Such, "pH‐responsive polymer nanoparticles for drug delivery," Macromolecular rapid communications, vol. 40, no. 10, p. 1800917, 2019.
S. Rafieian, H. Mirzadeh, H. Mahdavi, and M. E. Masoumi, "A review on nanocomposite hydrogels and their biomedical applications," Science and Engineering of Composite Materials, vol. 26, no. 1, pp. 154-174, 2019.
J. A. Kemp, and Y. J. Kwon, "Cancer nanotechnology: current status and perspectives," Nano convergence, vol. 8, no. 1, pp. 1-38, 2021.
R. Wei, S. Liu, S. Zhang, L. Min, and S. Zhu, "Cellular and extracellular components in tumor microenvironment and their application in early diagnosis of cancers," Analytical Cellular Pathology, vol. 2020, 2020.
G. Poornima, K. Harini, P. Pallavi, P. Gowtham, K. Girigoswami, and A. Girigoswami, "RNA–A choice of potential drug delivery system," International Journal of Polymeric Materials and Polymeric Biomaterials, pp. 1-15, 2022.
R. S. Kalhapure, and J. Renukuntla, "Thermo-and pH dual responsive polymeric micelles and nanoparticles," Chemico-Biological Interactions, vol. 295, pp. 20-37, 2018.
S. Thakkar, D. Sharma, K. Kalia, and R. K. Tekade, "Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review," Acta biomaterialia, vol. 101, pp. 43-68, 2020.
Z. Shi, Q. Li, and L. Mei, "pH-Sensitive nanoscale materials as robust drug delivery systems for cancer therapy," Chinese Chemical Letters, vol. 31, no. 6, pp. 1345-1356, 2020.
J. Kavya, G. Amsaveni, M. Nagalakshmi, K. Girigoswami, R. Murugesan, and A. Girigoswami, "Silver nanoparticles induced lowering of BCl2/Bax causes Dalton's Lymphoma tumour cell death in mice," Journal of Bionanoscience, vol. 7, no. 3, pp. 276-281, 2013.
R. Canaparo, F. Foglietta, F. Giuntini, C. Della Pepa, F. Dosio, and L. Serpe, "Recent developments in antibacterial therapy: Focus on stimuli-responsive drug-delivery systems and therapeutic nanoparticles," Molecules, vol. 24, no. 10, p. 1991, 2019.
A. A. Alghamdi, A. A. WalyEldeen, and S. A. Ibrahim, "Nano-particles as a therapeutic approach for tumor angiogenesis," Innovative Approaches for Nanobiotechnology in Healthcare Systems, pp. 52-113, 2022.
S. Ghosh, K. Girigoswami, and A. Girigoswami, "Membrane-encapsulated camouflaged nanomedicines in drug delivery," Nanomedicine, vol. 14, no. 15, pp. 2067-2082, 2019.
N. Piasentin, E. Milotti, and R. Chignola, "The control of acidity in tumor cells: a biophysical model," Scientific reports, vol. 10, no. 1, pp. 1-14, 2020.
G. Hao, Z. P. Xu, and L. Li, "Manipulating extracellular tumour pH: An effective target for cancer therapy," RSC advances, vol. 8, no. 39, pp. 22182-22192, 2018.
X. Pang, Y. Jiang, Q. Xiao, A. W. Leung, H. Hua, and C. Xu, "pH-responsive polymer–drug conjugates: Design and progress," Journal of controlled release, vol. 222, pp. 116-129, 2016.
Y. Li, "Multifunctional polymeric nanoparticles in targeted and controlled delivery for cancer therapy," Nanoengineering of Biomaterials, pp. 145-180, 2022.
F. Ofridam, M. Tarhini, N. Lebaz, E. Gagniere, D. Mangin, and A. Elaïssari, "pH‐sensitive polymers: Classification and some fine potential applications," Polymers for Advanced Technologies, vol. 32, no. 4, pp. 1455-1484, 2021.
N. Akhtar, P. -W. Wu, C. L. Chen, W. -Y. Chang, R. -S. Liu, C. T. Wu, A. Girigoswami, and S. Chattopadhyay, "Radiolabeled human protein-functionalized upconversion nanoparticles for multimodal cancer imaging," ACS Applied Nano Materials, Vol. 5, no. 5, pp. 7051-7062, 2022.
P. Sharmiladevi, K. Girigoswami, V. Haribabu, and A. Girigoswami, "Nano-enabled theranostics for cancer," Materials Advances, vol. 2, pp. 2876-2891, 2021.
R. Cheng, F. Meng, C. Deng, H.-A. Klok, and Z. Zhong, "Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery," Biomaterials, vol. 34, no. 14, pp. 3647-3657, 2013.
K. Liang, J. J. Richardson, H. Ejima, G. K. Such, J. Cui, and F. Caruso, "Peptide‐tunable drug cytotoxicity via one‐step assembled polymer nanoparticles," Advanced Materials, vol. 26, no. 15, pp. 2398-2402, 2014.
J. T. Wilson, S. Keller, M. J. Manganiello, C. Cheng, C. -C. Lee, C. Opara, A. Convertine, and P. S. Stayton, "pH-responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides," ACS nano, vol. 7, no. 5, pp. 3912-3925, 2013.
K. Zhou, Y. Wang, X. Huang, K. Luby‐Phelps, B. D. Sumer, and J. Gao, "Tunable, ultrasensitive pH‐responsive nanoparticles targeting specific endocytic organelles in living cells," Angewandte Chemie International Edition, vol. 50, no. 27, pp. 6109-6114, 2011.
Z.-b. Zhao, S.-s. An, H.-j. Xie, and Y. Jiang, "Copolymerization and properties of multicomponent crosslinked hydrogels," Chinese Journal of Polymer Science, vol. 33, no. 1, pp. 173-183, 2015.
Y.-L. Luo, W. Yu, and F. Xu, "pH-responsive PMAA-b-PEG-b-PMAA triblock copolymer micelles for prednisone drug release and release kinetics," Polymer bulletin, vol. 69, no. 5, pp. 597-620, 2012.
B. A. Abel, M. B. Sims, and C. L. McCormick, "Tunable pH-and CO2-responsive sulfonamide-containing polymers by RAFT polymerization," Macromolecules, vol. 48, no. 16, pp. 5487-5495, 2015.
P. D. Pickett, C. R. Kasprzak, D. T. Siefker, B. A. Abel, M. A. Dearborn, and C. L. McCormick, "Amphoteric, sulfonamide-functionalized “polysoaps”: CO2-induced phase separation for water remediation," Macromolecules, vol. 51, no. 21, pp. 9052-9059, 2018.
H. C. Kang, and Y. H. Bae, "pH‐tunable endosomolytic oligomers for enhanced nucleic acid delivery," Advanced Functional Materials, vol. 17, no. 8, pp. 1263-1272, 2007.
Q. -M. Wang, Z. Gao, S. Liu, B. Fan, L. Kang, W. Huang, and M. Jin, "Hybrid polymeric micelles based on bioactive polypeptides as pH-responsive delivery systems against melanoma," Biomaterials, vol. 35, no. 25, pp. 7008-7021, 2014.
Y. Yan, and H. Ding, "pH-responsive nanoparticles for cancer immunotherapy: a brief review," Nanomaterials, vol. 10, no. 8, p. 1613, 2020.
M. Nakayama, J. Akimoto, and T. Okano, "Polymeric micelles with stimuli-triggering systems for advanced cancer drug targeting," Journal of drug targeting, vol. 22, no. 7, pp. 584-599, 2014.
B. Nolting, "Linker technologies for antibody–drug conjugates," Antibody-drug conjugates, pp. 71-100, 2013.
T. Yoshida, T. C. Lai, G. S. Kwon, and K. Sako, "pH-and ion-sensitive polymers for drug delivery," Expert opinion on drug delivery, vol. 10, no. 11, pp. 1497-1513, 2013.
E. R. Gillies, A. P. Goodwin, and J. M. Fréchet, "Acetals as pH-sensitive linkages for drug delivery," Bioconjugate chemistry, vol. 15, no. 6, pp. 1254-1263, 2004.
E. M. Bachelder, T. T. Beaudette, K. E. Broaders, J. Dashe, and J. M. Fréchet, "Acetal-derivatized dextran: an acid-responsive biodegradable material for therapeutic applications," Journal of the American Chemical Society, vol. 130, no. 32, pp. 10494-10495, 2008.
S. L. Suarez, A. Muñoz, A. C. Mitchell, R. L. Braden, C. Luo, J. R. Cochran, A. Almutairi, and K .L. Christman, "Degradable acetalated dextran microparticles for tunable release of an engineered hepatocyte growth factor fragment," ACS biomaterials science & engineering, vol. 2, no. 2, pp. 197-204, 2016.
Y. Gu, Y. Zhong, F. Meng, R. Cheng, C. Deng, and Z. Zhong, "Acetal-linked paclitaxel prodrug micellar nanoparticles as a versatile and potent platform for cancer therapy," Bio-macromolecules, vol. 14, no. 8, pp. 2772-2780, 2013.
X. Huang, F. Du, J. Cheng, Y. Dong, D. Liang, S. Ji, S.-S. Lin, and Z. Li, "Acid-sensitive polymeric micelles based on thermoresponsive block copolymers with pendent cyclic orthoester groups," Macromolecules, vol. 42, no. 3, pp. 783-790, 2009.
R. Gannimani, P. Walvekar, V. R. Naidu, T. M. Aminabhavi, and T. Govender, "Acetal containing polymers as pH-responsive nano-drug delivery systems," Journal of controlled release, vol. 328, pp. 736-761, 2020.
B. Liu and S. Thayumanavan, "Substituent effects on the pH sensitivity of acetals and ketals and their correlation with encapsulation stability in polymeric nanogels," Journal of the American Chemical Society, vol. 139, no. 6, pp. 2306-2317, 2017.
J. Lai, Z. Xu, R. Tang, W. Ji, R. Wang, J. Wang, and C. Wang, "PEGylated block copolymers containing tertiary amine side-chains cleavable via acid-labile ortho ester linkages for pH-triggered release of DNA," Polymer, vol. 55, no. 12, pp. 2761-2771, 2014.
S. Li, L. Hu, D. Li, X. Wang, P. Zhang, J. Wang, G. Yan, and R. Tang, "Carboxymethyl chitosan-based nanogels via acid-labile ortho ester linkages mediated enhanced drug delivery," International journal of biological macromolecules, vol. 129, pp. 477-487, 2019.
R. Tang, W. Ji, D. Panus, R. N. Palumbo, and C. Wang, "Block copolymer micelles with acid-labile ortho ester side-chains: synthesis, characterization, and enhanced drug delivery to human glioma cells," Journal of controlled release, vol. 151, no. 1, pp. 18-27, 2011.
S. Aryal, C.-M. J. Hu, and L. Zhang, "Polymer− cisplatin conjugate nanoparticles for acid-responsive drug delivery," ACS nano, vol. 4, no. 1, pp. 251-258, 2010.
T. Senthilkumar, F. Lv, H. Zhao, L. Liu, and S. Wang, "Conjugated polymer nanogel binding anticancer drug through hydrogen bonds for sustainable drug delivery," ACS Applied Bio Materials, vol. 2, no. 12, pp. 6012-6020, 2019.
Y. Tao, S. Liu, Y. Zhang, Z. Chi, and J. Xu, "A pH-responsive polymer based on dynamic imine bonds as a drug delivery material with pseudo target release behavior," Polymer Chemistry, vol. 9, no. 7, pp. 878-884, 2018.
C.-W. Hsu, M.-H. Hsieh, M.-C. Xiao, Y.-H. Chou, T.-H. Wang, and W.-H. Chiang, "pH-responsive polymeric micelles self-assembled from benzoic-imine-containing alkyl-modified PEGylated chitosan for delivery of amphiphilic drugs," International Journal of Biological Macromolecules, vol. 163, pp. 1106-1116, 2020.
S. Zhou, S. Fu, H. Wang, Y. Deng, X. Zhou, W. Sun, and Y. Zhai, "Acetal-linked polymeric prodrug micelles based on aliphatic polycarbonates for paclitaxel delivery: Preparation, characterization, in vitro release and anti-proliferation effects," Journal of Biomaterials Science, Polymer Edition, vol. 31, no. 15, pp. 2007-2023, 2020.
K. E. Balan, C. Boztepe, and A. Künkül, "Modeling the effect of physical crosslinking degree of pH and temperature responsive poly (NIPAAm-co-VSA)/alginate IPN hydrogels on drug release behavior," Journal of Drug Delivery Science and Technology, vol. 75, p. 103671, 2022.
S. Nayak, K. Guleria, A. Sen, S. Banerjee, R. Subramanian, and P. Das, "Chemically induced crosslinked enhanced emission of carbon polymer dots discerning healthy and cancer cells through pH-dependent tunable photoluminescence," Journal of Materials Chemistry B, vol. 11, no. 3, pp. 594-605, 2023.
X. Wang, Y. Zheng, Y. Xue, Y. Wu, Y. Liu, X. Cheng, and R. Tang, "pH-sensitive and tumor-targeting nanogels based on ortho ester-modified PEG for improving the in vivo anti-tumor efficiency of doxorubicin," Colloids and Surfaces B: Biointerfaces, vol. 207, p. 112024, 2021.
P. Smyth, T. J. Gibson, G. Irvine, G. Black, D. Lavery, M. Semsarilar, C. J. Scott, and E. Themistou, "pH-Responsive benzaldehyde-functionalized PEG-based polymeric nanoparticles for drug delivery: Effect of preparation method on morphology, dye encapsulation and attachment," European Polymer Journal, vol. 124, p. 109471, 2020.
Y. Bobde, S. Biswas, and B. Ghosh, "PEGylated N-(2 hydroxypropyl) methacrylamide-doxorubicin conjugate as pH-responsive polymeric nanoparticles for cancer therapy," Reactive and Functional Polymers, vol. 151, p. 104561, 2020.
B. Fan, J. F. Trant, and E. R. Gillies, "End-capping strategies for triggering end-to-end depolymerization of polyglyoxylates," Macromolecules, vol. 49, no. 24, pp. 9309-9319, 2016.
M. Li, Z. Tang, S. Lv, W. Song, H. Hong, X. Jing, Y. Zhang, and X. Chen, "Cisplatin crosslinked pH-sensitive nanoparticles for efficient delivery of doxorubicin," Biomaterials, vol. 35, no. 12, pp. 3851-3864, 2014.
H. Feng, Y. Sun, J. Zhang, L. Deng, and A. Dong, "Influence of supramolecular layer-crosslinked structure on stability of dual pH-Responsive polymer nanoparticles for doxorubicin delivery," Journal of Drug Delivery Science and Technology, vol. 45, pp. 81-92, 2018.
S. Shahi, H. Roghani-Mamaqani, S. Talebi, and H. Mardani, "Stimuli-responsive destructible polymeric hydrogels based on irreversible covalent bond dissociation," Polymer Chemistry, 2022.
H. S. Han, T. Thambi, K. Y. Choi, S. Son, H. Ko, M. C. Lee, D .-G. Jo, Y. S. Chae, Y. M. Kang, and J. Y. Lee, "Bioreducible shell-cross-linked hyaluronic acid nanoparticles for tumor-targeted drug delivery," Biomacromolecules, vol. 16, no. 2, pp. 447-456, 2015.
G. Seetharaman, A. R. Kallar, V. M. Vijayan, J. Muthu, and S. Selvam, "Design, preparation and characterization of pH-responsive prodrug micelles with hydrolyzable anhydride linkages for controlled drug delivery," Journal of colloid and interface science, vol. 492, pp. 61-72, 2017.
B. Sarmento, and J. das Neves, Chitosan-based systems for biopharmaceuticals: delivery, targeting and polymer therapeutics. John Wiley & Sons, 2012.
J. Gou, Y. Liang, L. Miao, W. Guo, Y. Chao, H. He, Y. Zhang, J. Yang, C. Wu, and T. Yin, "Improved tumor tissue penetration and tumor cell uptake achieved by delayed charge reversal nanoparticles," Acta biomaterialia, vol. 62, pp. 157-166, 2017.
S. K. Hari, A. Gauba, N. Shrivastava, R. M. Tripathi, S. K. Jain, and A. K. Pandey, "Polymeric micelles and cancer therapy: An ingenious multimodal tumor-targeted drug delivery system," Drug Delivery and Translational Research, pp. 1-29, 2022.
V. Haribabu, P. Sharmiladevi, N. Akhtar, A. S. Farook, K. Girigoswami, and A. Girigoswami, "Label free ultrasmall fluoromagnetic ferrite-clusters for targeted cancer imaging and drug delivery," Current drug delivery, vol. 16, no. 3, pp. 233-241, 2019.
K. Harini, K. Girigoswami, and A. Girigoswami, "Nanopsychiatry: Engineering of nanoassisted drug delivery systems to formulate antidepressants," International Journal of Nano Dimension, 2022.
Y. Xu, L. Xiao, Y. Chang, Y. Cao, C. Chen, and D. Wang, "pH and redox dual-responsive MSN-SS-CS as a drug delivery system in cancer therapy," Materials, vol. 13, no. 6, p. 1279, 2020.
Q.-y. Wang, Q.-h. Hu, S.-h. Huang, J. Lin, and Q.-h. Zhou, "Surface charge switchable nano-micelle for pH/redox-triggered and endosomal escape mediated co-delivery of doxorubicin and paclitaxel in treatment of lung adenocarcinoma," Colloids and Surfaces B: Biointerfaces, p. 112588, 2022.
Q. Zhao, P. Xie, X. Li, Y. Wang, Y. Zhang, and S. Wang, "Magnetic mesoporous silica nanoparticles mediated redox and pH dual-responsive target drug delivery for combined magnetothermal therapy and chemotherapy," Colloids and Surfaces A: Physicochemical and Engineering Aspects, p. 129359, 2022.
M. Falsafi, N. Hassanzadeh Goji, A. Sh. Saljooghi, K. Abnous, S. M. Taghdisi, S. Nekooei, M. Ramezani, and M. Alibolandi, "Synthesis of a targeted, dual pH and redox-responsive nanoscale coordination polymer theranostic against metastatic breast cancer in vitro and in vivo," Expert Opinion on Drug Delivery, vol. 19, no. 6, pp. 743-754, 2022.
Y. Ding, C. Wang, Y. Ma, L. Zhu, B. Lu, Y. Wang, J. Wang, T. Chen, C.-M. Dong, and Y. Yao, "pH/ROS dual-responsive supramolecular polypeptide prodrug nanomedicine based on host-guest recognition for cancer therapy," Acta Biomaterialia, vol. 143, pp. 381-391, 2022.
Y. Li, M. Chen, B. Yao, X. Lu, B. Song, S. N. Vasilatos, X. Zhang, X. Ren, C. Yao, and W. Bian, "Dual pH/ROS‐responsive nanoplatform with deep tumor penetration and self‐amplified drug release for enhancing tumor chemotherapeutic efficacy," Small, vol. 16, no. 32, p. 2002188, 2020.
Z. Liu, S. Zhang, C. Gao, X. Meng, S. Wang, and F. Kong, "Temperature/pH-responsive carboxymethyl cellulose/poly (n-isopropyl acrylamide) interpenetrating polymer network aerogels for drug delivery systems," Polymers, vol. 14, no. 8, p. 1578, 2022.
N. V. Mdlovu, K.-S. Lin, M.-T. Weng, and Y.-S. Lin, "Design of doxorubicin encapsulated pH-/thermo-responsive and cationic shell-crosslinked magnetic drug delivery system," Colloids and Surfaces B: Biointerfaces, vol. 209, p. 112168, 2022.
T. Anirudhan, and J. Christa, "Temperature and pH sensitive multi-functional magnetic nanocomposite for the controlled delivery of 5-fluorouracil, an anticancer drug," Journal of Drug Delivery Science and Technology, vol. 55, p. 101476, 2020.
H. Zhao, and Y. Li, "A novel pH/temperature-responsive hydrogel based on tremella polysaccharide and poly (N-isopropyl-acrylamide)," Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 586, p. 124270, 2020.
H. Bera, Y. F. Abbasi, V. Gajbhiye, K. F. Liew, P. Kumar, P. Tambe, A. Azad, D. Cun, and M. Yang, "Carboxymethyl fenugreek galactomannan-g-poly (N-isopropylacrylamide-co-N,N′-methylene-bis-acrylamide)-clay based pH/temperature- responsive nanocomposites as drug-carriers," Materials Science and Engineering: C, vol. 110, p. 110628, 2020.
X. Wang, Y. Yang, C. Liu, H. Guo, Z. Chen, J. Xia, Y. Liao, C.-Y. Tang, and W.-C. Law, "Photo-and pH-responsive drug delivery nanocomposite based on o-nitrobenzyl functionalized upconversion nanoparticles," Polymer, vol. 229, p. 123961, 2021.
A. Pourjavadi, M. Kohestanian, and C. Streb, "pH and thermal dual-responsive poly(NIPAM-co-GMA)-coated magnetic nanoparticles via surface-initiated RAFT polymerization for controlled drug delivery," Materials Science and Engineering: C, vol. 108, p. 110418, 2020.
Y. Huang, Z. Tang, S. Peng, J. Zhang, W. Wang, Q. Wang, W. Lin, X. Lin, X. Zu, and H. Luo, "pH/redox/UV irradiation multi-stimuli responsive nanogels from star copolymer micelles and Fe3+ complexation for “on-demand” anticancer drug delivery," Reactive and Functional Polymers, vol. 149, p. 104532, 2020.
M. Su, S. Xiao, M. Shu, Y. Lu, Q. Zeng, J. Xie, Z. Jiang, and J. Liu, "Enzymatic multifunctional biodegradable polymers for pH-and ROS-responsive anticancer drug delivery," Colloids and Surfaces B: Biointerfaces, vol. 193, p. 111067, 2020.
D.-x. Ren, P.-c. Chen, P. Zheng, and Z.-n. Xu, "pH/redox dual response nanoparticles with poly-γ-glutamic acid for enhanced intracellular drug delivery," Colloids and Surfaces A: Physico-chemical and Engineering Aspects, vol. 577, pp. 412-420, 2019.
G. B. Demirel and Ş. Bayrak, "Ultrasound/redox/pH-responsive hybrid nanoparticles for triple-triggered drug delivery," Journal of Drug Delivery Science and Technology, vol. 71, p. 103267, 2022.
R. Zhang, R. Liu, C. Liu, L. Pan, Y. Qi, J. Cheng, J. Guo, Y. Jia, J. Ding, and J. Zhang, "A pH/ROS dual-responsive and targeting nanotherapy for vascular inflammatory diseases," Biomaterials, vol. 230, p. 119605, 2020.
W. Wu, L. Luo, Y. Wang, Q. Wu, H.-B. Dai, J.-S. Li, C. Durkan, N. Wang, and G.-X. Wang, "Endogenous pH-responsive nano-particles with programmable size changes for targeted tumor therapy and imaging applications," Theranostics, vol. 8, no. 11, p. 3038, 2018.
E.-K. Lim, B. H. Chung, and S. J. Chung, "Recent advances in pH-sensitive polymeric nanoparticles for smart drug delivery in cancer therapy," Current drug targets, vol. 19, no. 4, pp. 300-317, 2018.
S. Sur, A. Rathore, V. Dave, K. R. Reddy, R. S. Chouhan, and V. Sadhu, "Recent developments in functionalized polymer nanoparticles for efficient drug delivery system," Nano-Structures & Nano-Objects, vol. 20, p. 100397, 2019.
L. Palanikumar, S. Al-Hosani, M. Kalmouni, V.P. Nguyen, L. Ali, R. Pasricha, F. N. Barrera, and M. Magzoub, "pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics," Communications biology, vol. 3, no. 1, pp. 1-17, 2020.
P. Sadhukhan, M. Kundu, S. Chatterjee, N. Ghosh, P. Manna, J. Das, and P.C. Sil, "Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy," Materials science and engineering: C, vol. 100, pp. 129-140, 2019.
A. L. Harvey, R. Edrada-Ebel, and R. J. Quinn, "The re-emergence of natural products for drug discovery in the genomics era," Nature reviews drug discovery, vol. 14, no. 2, pp. 111-129, 2015.
M. S. Butler, A. A. Robertson, and M. A. Cooper, "Natural product and natural product derived drugs in clinical trials," Natural product reports, vol. 31, no. 11, pp. 1612-1661, 2014.
M. Kundu, P. Sadhukhan, N. Ghosh, S. Chatterjee, P. Manna, J. Das, and P.C. Sil, "pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized ZnO nano-particles for breast cancer therapy," Journal of advanced research, vol. 18, pp. 161-172, 2019.
A. Kostopoulou, and A. Lappas, "Colloidal magnetic nanocrystal clusters: Variable length-scale interaction mechanisms, synergetic functionalities and technological advantages," Nanotechnology Reviews, vol. 4, no. 6, pp. 595-624, 2015.
Y. Wang, Y. Zhang, J. Wang, and X.-J. Liang, "Aggregation-induced emission (AIE) fluorophores as imaging tools to trace the biological fate of nano-based drug delivery systems," Advanced Drug Delivery Reviews, vol. 143, pp. 161-176, 2019.
J. Lu, J. Sun, F. Li, J. Wang, J. Liu, D. Kim, C. Fan, T. Hyeon, and D. Ling, "Highly sensitive diagnosis of small hepatocellular carcinoma using pH-responsive iron oxide nanocluster assemblies," Journal of the American Chemical Society, vol. 140, no. 32, pp. 10071-10074, 2018.
G.-R. Tan, C.-Y. S. Hsu, and Y. Zhang, "pH-responsive hybrid nanoparticles for imaging spatiotemporal ph changes in biofilm-dentin microenvironments," ACS Applied Materials & Interfaces, vol. 13, no. 39, pp. 46247-46259, 2021.
D. Ding, K.-Y. Pu, K. Li, and B. Liu, "Conjugated oligo-electrolyte-polyhedral oligomeric silsesquioxane loaded pH-responsive nanoparticles for targeted fluorescence imaging of cancer cell nucleus," Chemical Communications, vol. 47, no. 35, pp. 9837-9839, 2011.
M. Theodosiou, N. Boukos, E. Sakellis, M. Zachariadis, and E. K. Efthimiadou, "Gold nanoparticle decorated pH-sensitive polymeric nanocontainers as a potential theranostic agent," Colloids and Surfaces B: Biointerfaces, vol. 183, p. 110420, 2019.
A. Saha, S. C. Mohanta, K. Deka, P. Deb, and P. S. Devi, "Surface-engineered multifunctional Eu: Gd2O3 nanoplates for targeted and pH-responsive drug delivery and imaging applications," ACS applied materials & interfaces, vol. 9, no. 4, pp. 4126-4141, 2017.
Y.-T. Qin, H. Peng, X.-W. He, W.-Y. Li, and Y.-K. Zhang, "pH-responsive polymer-stabilized ZIF-8 nanocomposites for fluorescence and magnetic resonance dual-modal imaging- guided chemo-/photodynamic combinational cancer therapy," ACS applied materials & interfaces, vol. 11, no. 37, pp. 34268-34281, 2019.
M. S. Foroushani, N. Niroumand, R. K. Shervedani, F. Yaghoobi, A. Kefayat, and M. Torabi, "A theranostic system based on nanocomposites of manganese oxide nanoparticles and a pH sensitive polymer: Preparation, and physicochemical characterization," Bioelectrochemistry, vol. 130, p. 107347, 2019.
H. Xiao, X. Li, C. Zheng, Q. Liu, C. Sun, J. Huang, Y. Wang, and Y. Yuan, "Intracellular pH-responsive polymeric micelle for simultaneous chemotherapy and MR imaging of hepatocellular carcinoma," Journal of Nanoparticle Research, vol. 22, no. 5, pp. 1-15, 2020.
Y. Esmaeili, M. Khavani, A. Bigham, A. Sanati, E. Bidram, L. Shariati, A. Zarrabi, N.A. Jolfaie, and M. Rafienia, "Mesoporous silica@ chitosan@ gold nanoparticles as “on/off” optical biosensor and pH-sensitive theranostic platform against cancer," International Journal of Biological Macromolecules, vol. 202, pp. 241-255, 2022.
P. Kumar, T. Van Treuren, A. P. Ranjan, P. Chaudhary, and J. K. Vishwanatha, "In vivo imaging and biodistribution of near infrared dye loaded brain-metastatic-breast-cancer-cell-membrane coated polymeric nanoparticles," Nanotechnology, vol. 30, no. 26, p. 265101, 2019.
C. Zhang, J. Li, C. Yang, S. Gong, H. Jiang, M. Sun, and C. Qian, "A pH-sensitive coordination polymer network-based nanoplatform for magnetic resonance imaging-guided cancer chemo-photothermal synergistic therapy," Nanomedicine: Nanotechnology, Biology and Medicine, vol. 23, p. 102071, 2020.
Z. Li, Q. Yin, B. Chen, Z. Wang, Y. Yan, T. Qi, W. Chen, Q. Zhang, and Y. Wang, "Ultra-pH-sensitive indocyanine green-conjugated nanoprobes for fluorescence imaging-guided photo-thermal cancer therapy," Nanomedicine: Nanotechnology, Biology and Medicine, vol. 17, pp. 287-296, 2019.
Y. Wang, X. Xiong, Y. Zhu, X. Song, Q. Li, and S. Zhang, "A pH-Responsive nanoplatform based on fluorescent conjugated polymer dots for imaging-guided multitherapeutics delivery and combination cancer therapy," ACS Biomaterials Science & Engineering, vol. 8, no. 1, pp. 161-169, 2021.
C. Zhou, Q. Yang, X. Zhou, and N. Jia, "PDA-coated CPT@ MIL-53 (Fe)-based theranostic nanoplatform for pH-responsive and MRI-guided chemotherapy," Journal of Materials Chemistry B, vol. 10, no. 11, pp. 1821-1832, 2022.
ดาวน์โหลด
เผยแพร่แล้ว
วิธีการอ้างอิง
ฉบับ
บท
การอนุญาต
ลิขสิทธิ์ (c) 2023 วารสารโลหะ, วัสดุ และแร่

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












