Synthesis, characterization, and application of ZIF-8 for removal of Cd, Ni, and Pb ions from aqueous solutions: Optimization of the process by Response Surface Methodology (RSM) based on Central Composite Design (CCD) technique
DOI:
https://doi.org/10.55713/jmmm.v33i2.1668คำสำคัญ:
Adsorption, Optimized parameter, health risk, heavy metals, transfer factor, vegetables, zeolite imidazolate framework, central composite design, response surface methodology, Water treatment, Characterizationบทคัดย่อ
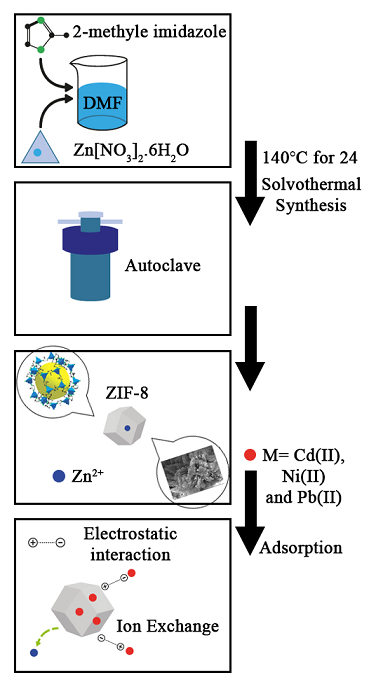
A Zeolitic imidazolate framework-8 (ZIF-8) was synthesized by the solvothermal method of zinc nitrate hexahydrate and 2-methylimidazole in DMF to remove Cd(II), Ni(II), and Pb(II) ions from aqueous solutions. The synthesized ZIF-8 was distinguished by XRD, FT-IR, BET, SEM, EDX, TEM methods. Several significant variables were optimized with response surface methodology (RSM) to obtain the highest removal of metal ions. According to the achieved results, the aqueous solution pH values of 6.5, 6.5, and 6.0, ZIF-8 dosages of 0.05, 0.06, and 0.05 g⸳L-1, and metal ions initial concentrations of 50, 60, and 60 mg⸳L-1 were chosen as the optimum amount of these variables for Cd(II), Ni(II), and Pb(II) ions adsorption from solution, respectively. The equilibrium time for metal ions adsorption was found at 50 min. Three-dimensional plots demonstrate relationships between the metal ion uptakes with the paired factors, which illustrate the behavior of the sorption system in a batch process. Based on the experimental results and model parameters, maximum adsorption efficiencies were achieved 89.76, 72 and 68.43% for Cd(II), Ni(II) and Pb(II), respectively. It can be suggested that the synthetized ZIF-8 has excellent potential as an effective adsorbent and used for heavy metal sorption from water environment.
Downloads
เอกสารอ้างอิง
D. Witkowska, J. Słowik, and K. Chilicka, “Heavy metals and human health: Possible exposure pathways and the competition for protein binding sites,” Molecules. vol. 26, pp. 1-16, 2021.
H. A. Al-Swadi, A. R. Usman, A. S. Al-Farraj, M.I. Al-Wabel, M. Ahmad, and A. Al-Faraj, “Sources, toxicity potential, and human health risk assessment of heavy metals-laden soil and dust of urban and suburban areas as affected by industrial and mining activities,” Scientific Reports, vol, 121, pp.1-18, 2022.
M. Balali-Mood, K. Naseri, Z. Tahergorabi, M.R. Khazdair, M. Sadeghi, “Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic,” Frontiers in pharmacology, vol, 12, p. 643972, 2021.
R.K. Sharma, and M. Agrawal, “Biological Effects of Heavy Metals: An Overview,” Journal of Environmental Biology, vol. 262, pp. 301-313, 2005.
K. Li, N. Miwornunyuie, L. Chen, H. Jingyu, P. S. Amaniampong, D. Ato Koomson, D. Ewusi-Mensah, W. Xue, G. Li, and H. Lu, “Sustainable application of ZIF-8 for heavy-metal removal in aqueous solutions,” Sustainability, vol. 13, no. 2, p. 984, 2021.
R. Abdeldayem, “A preliminary study of heavy metals pollution risk in water,” Applied Water Science, vol. 101, pp. 1-4, 2020.
E. B. Silva, I. S. Alves, L. R. F. Alleoni, P. H. Grazziotti, M. M. M. Farnezi, L. L. Santos, J. T. Prochnow, and I. C. I. Fontan, “Availability and toxic level of cadmium, lead and nickel in contaminated soils,” Communications in Soil Science and Plant Analysis, vol. 51, no. 10, pp. 1341-1356, 2020.
N. A. Qasem, R. H. Mohammed, and D. U. Lawal, “Removal of heavy metal ions from wastewater: A comprehensive and critical review,” Npj Clean Water, vol. 4, no. 1, pp. 1-15, 2021.
B. Chen, Z. Yang, Y. Zhu, and Y. Xia, “Zeolitic imidazolate framework materials: recent progress in synthesis and applications,” Journal of Materials Chemistry A, vol. 2, no. 40, pp. 16811-16831, 2014.
Y. Zhang, Z. Xie, Z. Wang, X. Feng, Y. Wang, and A. Wu, “Unveiling the adsorption mechanism of zeolitic imidazolate framework-8 with high efficiency for removal of copper ions from aqueous solutions,” Dalton Transactions, vol. 45, no. 32, pp.12653-12660, 2016.
M. Niknam Shahrak, M. Ghahramaninezhad, and M. Eydifarash, “Zeolitic imidazolate framework-8 for efficient adsorption and removal of Cr(VI) ions from aqueous solution,” Environmental Science and Pollution Research, vol. 24, no. 10, pp. 9624-9634, 2017.
Y. Zhang, Z. Xie, Z. Wang, X. Feng, Y. Wang, and A. Wu, “Unveiling the adsorption mechanism of zeolitic imidazolate framework-8 with high efficiency for removal of copper ions from aqueous solutions,” Dalton Transactions, vol. 45, no. 32, pp. 12653-12660, 2016.
A. Tanihara, K. Kikuchi, and H. Konno, “Insight into the mechanism of heavy metal removal from water by mono-disperse ZIF-8 fine particles,” Inorganic Chemistry Communications, vol. 131, p. 108782, 2021.
Y. Chen, and S. Tang, “Solvothermal synthesis of porous hydrangea-like zeolitic imidazole framework-8 (ZIF-8) crystals,” Journal of Solid State Chemistry, vol. 276, pp. 68-74, 2019.
A. A. Tezerjani, R. Halladj, and S. Askari, “Different view of solvent effect on the synthesis methods of zeolitic imidazolate framework-8 to tuning the crystal structure and properties,” RSC advances, vol. 11, no. 32, pp. 19914-19923, 2021.
F. Hillman, J.M. Zimmerman, S.M. Paek, M.R. Hamid, W.T. Lim, H.K. Jeong, “Rapid microwave-assisted synthesis of hybrid zeolitic–imidazolate frameworks with mixed metals and mixed linkers,” Journal of Materials Chemistry A, vol. 5, no. 13, pp. 6090-6099, 2017.
S. Nalesso, G. Varlet, M. J. Bussemaker, R. P. Sear, M. Hodnett, R. Monteagudo-Oliván, V. Sebastián, J. Coronas, and J. Lee, “Sonocrystallisation of ZIF-8 in water with high excess of ligand: Effects of frequency, power and sonication time,” Ultrasonics Sonochemistry, vol. 76, p. 105616, 2021.
P. Ji, R. Tian, H. Zheng, J. G. Jiang, J. Sun, and J. Peng, “Solvent-free synthesis of ZIF-8 from zinc acetate with the assistance of sodium hydroxide,” Dalton Transactions, vol. 49, no. 36, pp. 12555-12558, 2020.
P. Ji, X. Hu, R. Tian, H. Zheng, J. Sun, W. Zhang, and J. Peng. “Atom-economical synthesis of ZnO@ ZIF-8 core–shell heterostructure by dry gel conversion (DGC) method for enhanced H2 sensing selectivity,” Journal of Materials Chemistry C, vol. 8, no. 8, pp. 2927-2936, 2020.
O. Kolmykov, J.M. Commenge, H. Alem, E. Girot, K. Mozet, G. Medjahdi, and R. Schneider, “Microfluidic reactors for the size-controlled synthesis of ZIF-8 crystals in aqueous phase,”
Materials & Design, vol. 122, pp. 31-41, 2017.
K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O’Keeffe, and O. M. Yaghi, “Exceptional chemical and thermal stability of zeolitic imidazolate frameworks,” Proceedings of the National Academy of Sciences, vol. 103, no. 27, pp. 10186-10191, 2006.
T. Lundstedt, E. Seifert, L. Abramo, B. Thelin, A. Nystrom, J. Pettersen, and R. Bergman, “Experimental design and optimization,” Chemometrics and Intelligent Laboratory Systems, vol. 42, no. 1-2, pp. 3-40, 1998.
A. Khosravi, M. Esmhosseini, J. Jalili, and S. Khezri, “Optimization of ammonium removal from waste water by natural zeolite using central composite design approach,” Journal of Inclusion Phenomena and Macrocyclic Chemistry, vol. 74, pp. 383-390, 2012.
H. Y. Jang, J. K. Kang, J. A. Park, S. C. Lee, and S. B. Kim,” Metal-organic framework MIL-100(Fe) for dye removal in aqueous solutions: Prediction by artificial neural network and response surface methodology modeling,” Environmental Pollution, vol. 267, p. 115583, 2020.
M. Darvishmotevalli, A. Zarei, M. Moradnia, M. Noorisepehr, and H. Mohammadi, “Optimization of saline wastewater treatment using electrochemical oxidation process: prediction by rsm Method,” MethodsX, vol. 6, pp. 1101–1113, 2019.
J. Cravillon, S. Münzer, S. J. Lohmeier, A. Feldhoff, K. Huber, and M. Wiebcke, “Rapid room-temperature synthesis and characterization of nanocrystals of a prototypical zeolitic imidazolate framework,” Chemistry of Materials, vol. 21, no. 8, pp. 1410-1412, 2009.
Y. J. Zhang, Z. Q. Xie, Z. Q. Wang, X. H. Feng, Y. Wang, and A. G. Wu, “Unveiling the adsorption mechanism of zeolitic imidazolate framework-8 with high efficiency for removal of copper ions from aqueous solutions,” Dalton Transactions, vol. 45, no. 32, pp. 12653-12660, 2016.
Z. Lian, L. Huimin, and O. Zhaofei, “In situ crystal growth of zeolitic imidazolate frameworks (ZIF) on electrospun poly-urethane nanofibers,” Dalton Trans, vol. 43, pp. 6684-6688, 2014.
U. P. N. Tran, K. K. A. Le, and N. T. S. Phan, “Expanding applications of metal organic frameworks: zeolite imidazolate framework zif-8 as an efficient heterogeneous catalyst for the knoevenagel reaction,” ACS Catalysis, vol. 1, pp. 120-127, 2011.
C. S. Wu, Z .H. Xiong, C. Li, and J. M. Zhang, “Zeolitic imidazolate metal organic framework ZIF-8 with ultra-high adsorption capacity bound tetracycline in aqueous solution,” RSC Advances, vol. 5, pp. 82127-82137, 2015.
H. L. Jiang, B. Liu, T. Akita, M. Haruta, H. Sakurai, and Q. J. Xu, “Au@ZIF-8: CO oxidation over gold nanoparticles deposited to metal-organic framework,” Journal of the American Chemical Society, vol. 131, pp. 11302-11303, 2009.
Z. Öztürk, M. Filez, and B. M. Weckhuysen, “Decoding nucleation and growth of zeolitic imidazolate framework thin films with atomic force microscopy and vibrational spectroscopy,” Chemistry–A European Journal, vol. 23, no. 45, pp. 10915-10924, 2017.
Y. Ding, Y. Xu, B. Ding, Z. Li, F. Xie, F. Zhang, H. Wang, J. Liu, and X. Wang, “Structure induced selective adsorption performance of ZIF-8 nanocrystals in water,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 520, pp. 661-667, 2017,
E. Binaeian, S. Maleki, N. Motaghedi, and M. Arjmandi, “Study on the performance of Cd2+ sorption using dimethyl -ethylenediamine-modified zinc-based MOF (ZIF-8-mmen): optimization of the process by RSM technique,” Separation Science and Technology, vol. 55, pp. 2716-2728, 2020.
Y. J. Zhang, Z. Q. Xie, Z .Q. Wang, X. H. Feng, Y. Wang, and A. G. Wu, “Unveiling the adsorption mechanism of zeolitic imidazolate framework-8 with high efficiency for removal of copper ions from aqueous solutions,” Dalton Transactions, vol. 45, no. 32, pp. 12653-12660, 2016.
S. J. Hoseini, M. Bahrami, and S. M. Nabavizadeh, “ZIF-8 nanoparticles thin film at an oil–water interface as an electro-catalyst for the methanol oxidation reaction without the application of noble metals,” New Journal of Chemistry, vol. 43, pp. 15811-15822, 2019.
Y. Pan, Y. Liu, G. Zeng, L. Zhao, and Z. Lai, “Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system,” Communications chemistry, vol. 47, pp. 2071-2073, 2011.
A. F. Gross, E. Sherman, and J. J. Vajo, “Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks,” Dalton Transactions, vol. 41, pp. 5458-5460, 2012.
H. Esfandian, A. Akrami, and F. B. Shahri, “Application of response surface methodology (RSM) for optimization of strontium sorption by synthetic ppy/perlite nanocomposite,” Desalination and Water Treatment, vol. 93, pp. 74-82, 2017.
F. N. Chianeh, and J. B. Parsa, “Electrochemical degradation of metronidazole from aqueous solutions using stainless steel anode coated with SnO2 nanoparticles: experimental design,” Journal of the Taiwan Institute of Chemical Engineers, vol. 59, pp. 424-432, 2016.
S. Wang, L. Bromberg, H. Schreuder-Gibson, and T. A. Hatton, “Organophophorous ester degradation by chromium (III) terephthalate metal–organic framework (MIL-101) chelated to N, N-Dimethylaminopyridine and related aminopyridines,” ACS Applied Materials & Interfaces, vol. 5, pp. 1269-1278, 2013.
A. J. Howarth, Y. Liu, P. Li, Z. Li, T. C. Wang, J. Hupp, and O. Farha, “Thermal and mechanical stabilities of metal-organic frameworks,” Nature Reviews Materials, vol. 3, pp. 1-15, 2016.
M. P. Jian, B. Liu, G. S. Zhang, R. P. Liu, and X. W. Zhang, “Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, vol. 465, pp. 67-76, 2014.
M. Roushani, Z. Saedi, amd Y. Baghelani, “Removal of cadmium ions from aqueous solutions using TMU-16-NH2 metal organic framework,” Environmental Nanotechnology, Monitoring and Management, vol. 7, pp. 89-96, 2017.
A. Heidari, H. Younesi, and Z. Mehraban, “Removal of Ni(II), Cd(II), and pb(II) from a ternary aqueous solution by amino functionalized mesoporous and nanomesoporous silica”, Chemical Engineering Journal, vol. 153, pp. 70-79, 2009.
Y. X. Li, H. N. Zhang, Y. T. Chen, L. Huang, Z. A. Lin, and Z. W. Cai, “Core-shell structured magnetic covalent organic framework nanocomposites for triclosan and triclocarban adsorption,” ACS Applied Materials & Interfaces, vol, 11, pp. 22492-22500, 2019.
C. G. Lee, J. W. Jeon, M. J. Hwang, K. H. Ahn, C. Park, J. W. Choi, and S. H. Lee, “Lead and copper removal from aqueous solutions using carbon foam derived from phenol resin,” Chemosphere, vol. 130, pp. 59-65, 2015.
E. Cerrahoğlu, A. Kayan, and D. Bingöl, “Multivariate optimization for removal of some heavy metals using novel inorganic–organic hybrid and calcined materials,” Separation Science and Technology, vol. 53, pp. 2563-2572, 2018.
M. R. Awual, “A novel facial composite adsorbent for enhanced copper(II) detection and removal from wastewater,” Chemical Engineering Journal, vol. 266, pp. 368-375, 2015.
S. Kushwaha, H. Soni, V. Ageetha, and P. Padmaja, “An insight into the production, characterization, and mechanisms of action of low-cost adsorbents for removal of organics from aqueous solution,” Critical Reviews in Environmental Science and Technology , vol. 43, pp. 443-549, 2013.
L. Ma, X. Zhang, M. Ikram, M. Ullah, H. Wu, and K. Shi, “Controllable synthesis of an intercalated ZIF-67/EG structure for the detection of ultratrace Cd2+, Cu2+, Hg2+ and Pb2+ ions,” Chemical Engineering Journal, vol. 395, p. 125216, 2020.
ดาวน์โหลด
เผยแพร่แล้ว
วิธีการอ้างอิง
ฉบับ
บท
การอนุญาต
ลิขสิทธิ์ (c) 2023 วารสารโลหะ, วัสดุ และแร่

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












