Bio-composite of nipa palm husk derived activated carbon/poly(butylene succinate): an effective agricultural waste based adsorbent for ammonia removal
DOI:
https://doi.org/10.55713/jmmm.v32i1.1248Keywords:
Biodegradable polymer, Activated carbon, Nipa palm husk, Poly(butylene succinate), Ammonia adsorptionAbstract
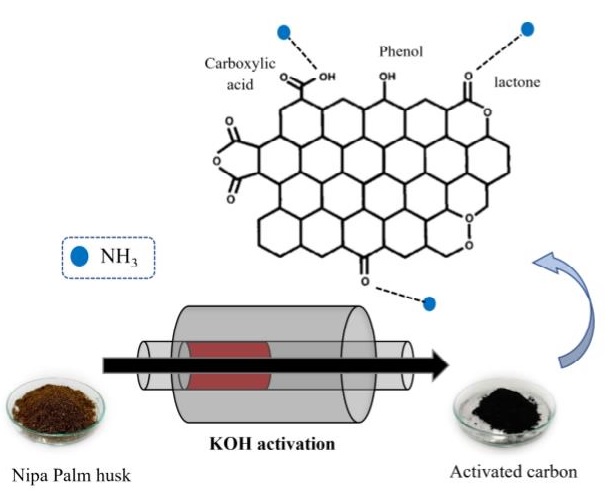
In this work, activated materials were successfully prepared by pyrolyzing various ratios of Nipa palm husk powder to potassium hydroxide (1:1, 1:2, and 1:3) at predetermined temperatures (500℃, 600℃, and 700℃). The surface area was obtained from nitrogen isotherms using the Brunauer-Emmett-Teller (BET) equation. The surface area of the prepared activated carbon was increased with decreasing potassium hydroxide impregnation ratio and increasing pyrolyzing temperature. The highest surface area was 1,211 m2×g-1. In contrast, acidic surface functional groups investigated by Boehm’s titration were increased with decreasing pyrolyzing temperature and impregnation ratio. Ammonia removal capacity was increased with an increase in the acidic surface functional groups on prepared activated carbon. Therefore, the activated sample with the highest acidic surface functional groups contents provided the greatest ammonia adsorption of around 95% (from 100 ppm to 5 ppm), implying that ammonia removal capacity was closely related to the acidic surface functional groups on the activated carbon. Adsorptive poly(butylene succinate) was carried out by incorporating activated carbon into the poly(butylene succinate) matrix for ammonia adsorption. This adsorption increased with increasing activated carbon content so that it was found that 12 wt% activated carbon provided the highest ammonia adsorption by reducing ammonia from 108 ppm to 26 ppm.
Downloads
References
N. F. Attia, M. A. Diab, A. S. Attia, and M. F. El-Shahat, “Greener approach for fabrication of antibacterial graphene-polypyrrole nanoparticle adsorbent for removal of Mn2+ from aqueous solution,” Synthetic Metals, vol. 282, p. 116951, 2021.
A. Galal, M. M. Zaki, N. F. Atta, S. H. Samaha, H. E. Nasr, and N. F. Attia, “Electroremoval of copper ions from aqueous solutions using chemically synthesized polypyrrole on polyester fabrics,” Journal of Water Process Engineering, vol. 43, p. 102287, 2021.
J. Park, S. Y. Cho, M. Jung, K. Lee, Y. C. Nah, N. F. Attia, and H. Oh, “Efficient synthetic approach for nanoporous adsorbents capable of pre- and post-combustion CO2,” Journal of CO2 Utilization, vol. 45, p. 101404, 2021.
M. A. Diab, N. F. Attia, A. S. Attia, and M. F. El-Shahat, “Green synthesis of cost-effective and efficient nanoadsorbents based on zero and two dimensional nanomaterials Zn2+ and Cr3+ removal from aqueous solutions,” Synthetic Metals, vol. 265, p. 116411, 2020.
J. Park, T. Kang, Y. Heo, K. Lee, K. Kim, K. Lee, and C. Yoon, “Evaluation of short-term exposure levels on ammonia and hydrogen sulfide during manure-handling processes at livestock farms,” Safety and Health at Work, vol. 11, pp. 109-117, 2020.
M. A. Farea, H. Y. Mohammed, S. M. Shirsat, P. W. Sayyad, N. N. Ingle, T. Al-Gahouari, M. M. Mahadik, G. A. Bodkhe, and M. D. Shirsat, “Hazardous gases sensors based on conducting polymer composites: Review,” Chemical Physics Letters, vol. 776, p. 138703, 2021.
S. J. Blonigen, A. G. Fassbender, R. D. Litt, B. F. Monzyk, and R. Neff, “Apparatus and method for ammonia removal from waste streams,” U.S. patent 6,838,069, published Jan 4, 2005.
M. Danish, R. Hashim, M. N. M. Ibrahim, and O. Sulaiman, “Effect of acidic activating agents on area and surface functional groups of activated carbons produced from Acacia mangium wood,” Journal of Anaytical and Applied Pyrolysis, vol. 104, pp. 418-425, 2013.
X. Wang, H. Cheng, G. Ye, J. Fan, F. Yao, Y. Wang, Y. Jiao, W. Zhu, H. Huang, and D. Ye, “Key factors and primary modification methods of activated carbon and their application in adsorption of carbon-based gases: A review,” Chemosphere, vol. 287, no. 2, p. 131995, pp. 1-19, 2022.
C. A. Toles, W. E. Marshall, and M. M. Johns, “Surface functional groups on acid-activated nutshell carbons,” Carbon, vol. 37, pp. 1207, 1999.
B. J. Kim, and S. J. Park, “Effects of carbonyl group formation on ammonia adsorption of porous carbon surfaces,” Journal of Colloid and Interface Science, vol. 311, pp. 311-314, 2007.
J. Guo, W. S. Xu, Y. L. Chen, and A.C. Lua, “Adsorption of NH3 onto activated carbon prepared from palm shells impregnated with H2SO4,” Journal of Colloid and Interface Science, vol. 281, pp. 285-290, 2005.
A. J. Allen, L.Whitten, and G. Mckay, “The production and characterization of activared carbons: A review,” Developments in Chemical Engineering and Mineral Process, vol. 6, pp. 2311-261, 1988.
S. Kim, S. Y. Cho, K. Son, N. F. Attia, and H. Oh, “A metal-doped flexible porous carbon cloth for enhanced CO2/CH4 separation,” Separation and Purification Technology, vol. 227, p. 119511, 2021.
M. Jung, J. Park, S. Y. Cho, S. E. A. Elashery, N. F. Attia, and H. Oh, “Flexible carbon sieve based on nanoporous carbon cloth for efficient CO2/CH4 separation,” Surfaces and Interfaces vol. 23, p. 100960, 2021.
M. Jung, J. Park, K. Lee, N. F. Attia, and H. Oh, “Effective synthesis route of renewable nanoporous carbon adsorbent for high energy storage and CO2/N2 selectivity,” Renewable Energy, vol.161, pp. 30-42, 2020.
A. Demirbas, “Agricultural based activated carbons for the removal of dyes from aqueous solutions: A review,” Journal of Hazardous Materials, vol. 167, pp. 1-9, 2009.
D. Sud, G. Mahajan, and M. P. Kaur, “Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions-A review,” Bioresource Technology, vol. 99, pp. 6017-6027, 2008.
M. Baysal, K. Bilge, B. Yılmaz, M. Papila, and Y. Yürüm, “Preparation of high surface area activated carbon from waste-biomass of sunflower piths: Kinetics and equilibrium studies on the dye removal,” Journal of Environmental Chemical Engineering, vol. 6, issue 2, pp. 1702-1713, 2018.
P. Tamunaidu, and S. Saka, “Chemical characterization of various parts of nipa palm (Nypa fruticans),” Industrial Crops and Products, vol. 34, no. 3, pp. 1423-1428, 2011.
V. Sricharoenchaikul, C. pechyen, D. Aht-ong, and D. Atong, “Preparation and characterization of activated carbon from the pyrolysis of physic nut (Jatropha curcus L.),” Energy and Fuels, vol. 22, no. 1, pp. 31-37, 2008.
S. Brunauer, S. Emmett, and F. Teller, “Adsorption of gases in multimolecular layers,” Journal of the American Chemical Society, vol. 60, pp. 309-319, 1951.
H. P. Boehm, “Some aspects of the surface chemistry of carbon blacks and other carbons,” Carbon, vol. 32, pp. 759-769, 1994.
C. C. Huang, H. S. Li, C. H. Chen, “Effect of surface acidic oxides of activated carbon on of ammonia,” Journal of Hazardous Materials, vol. 159, pp. 523-527, 2008.
X. Jin, Z. Yu, and Y. U.Wu, “Preparation of activated carbon from lignin obtained by straw pulping by KOH and K2CO3,” Cellulose Chemistry and Technology, vol. 46, pp. 79-85, 2012.
R. L. Tseng, S. K. Tseng, F. C. Wu, C. C. Hu, and C. C. Wang, “Effects of micropore development on the physicochemical properties of KOH-activated carbons,” Journal of the Chinese Institute of Chemical Engineers, vol. 39, no. 1, pp. 37-47, 2008.
S. Mopoung, P. Moonsiri, W. Palas, and S. Khumpai, “Characterization and properties of activated carbon prepared from tarmarind seeds by KOH activation for Fe(III) adsorption from aqueous solution,” The Scientific World Journal, 2015.
A. Garcίa-Garcίa, A. Gregόrio, C. Franco, F. Pinto, D. Boavida, and I. Gulyurtlu, “Unconverted chars obtained during biomass gasification on a pilot scale gasifier as a source of activated carbon production,” Bioresource Technology, vol. 88, pp. 27-32, 2003.
S. J. Park, and W. Y. Jung, “Preparation and structural characterization of activated carbons based on polymeric resin,” Journal of Colloid and Interface Science, vol. 250, pp. 196-200, 2002.
K. L. Van, and T. T. L. Thi, “Activated carbon derived from rice husk by NaOH activation and its application in supercapacitor,” Progress in Natural Science: Materials International, vol. 24, pp. 191-198, 2014.
J. L. Figueiredo, M. F. R. Pereira, M. M. A. Freitas, and J. J. M. Orfao, “Modifications of the surface chemistry of activated carbons,” Carbon, vol. 37, pp. 1379-1389, 1999.
T. Mochizuki, M. Kubota, H. Matsuda, and L. F. D’Elia Camacho, “Adsorption behaviors of ammonia and hydrogen sulfide on activated carbon prepared from petroleum coke by KOH chemical activation,” Fuel Processing Technology, vol. 144, pp. 164-169, 2016.
N. A. Travlou, M. Seredych, E. Rodriguez-Castellon, and T. J. Bandosz, “Activated carbon-based gas sensors: effects of surface features on the sensing mechanism,” Journal of Materials A, vol. 3, pp. 3821-3831, 2015.
B. Han, W. zhang, J. Z. He, and D. Chen, “Lignite ammonia adsorption and surface chemistry after dewatering,” Separation and Purification Technology, vol. 253, 117483, 2020.
M. Seredych, and T. J. Bandosz, “Mechanism of ammonia retention on graphite oxides: Role of surface chemistry and structure,” The Journal of Physical Chemistry C, vol. 111, pp. 15596-15604, 2007.
W. Zheng, J. Hu, S. Rappeport, Z. Zheng, Z. Wang, Z. Han, J. Langer, and J. Economy, “Activated carbon fiber composites for gas phase ammonia adsorption,” Microporous and Mesoporous, vol. 234, pp. 146-154, 2016.
Y. Gao, O. T. Picot, H. Zhang, E. Bilotti, and T. Peus, “Synergistic effects of filler size on thermal annealing-induced percolation in polylactic acid (PLA/Graphite nanoplatelet (GNP nanocomposites,” Nanocomposites, vol. 3, no. 2, pp. 67-75, 2017.
M. El. Achaby, F. E. Arrakhiz, S. Vaudreuil, Q. El Kacem, M. A. Bousmina, and O. Fassi-Fehri, “Mechanical, thermal, and rheological properties of graphene-based polypropylene nanocomposites prepared by melt mixing,” Polymer Composites, vol. 33, pp. 733-744, 2012.
L. W. Xiong, and K. H. Badri, “Preparation of polyurethane composited with activated carbon z black as the reinforcing filler,” Journal of Polymer Science and Technology, vol. 3, no. 1, pp. 11-18, 2018.
M. A. Herrera, A. P. Mathew, and K. Oksman, “Gas permeability and selectivity of cellulose nanocrystals films(layers) deposited by spin coating,” Carbohydrate. Polymer, vol. 112, pp. 494-501, 2014.
R. David, and A. W. Neumann, “A theory for surface tensions and contact angles of hydrogen-bonding liquids,” Langmuir, vol. 30, no. 39, pp. 11634-11639, 2014.
N. Spahis, M. Dellali, and H. Mahmoudi, “Synthesis and characterization of polymeric/activated carbon membranes,” Procedia Engineering, vol. 33, pp. 47-51, 2012.
X. L. Long, H. Cheng, X. L. Xin, W. D. Xiao, W. Li, and W. K. Yuan, “Adsorption of ammonia on activated carbon form aqueous solutions,” Environmental Progress, vol. 27, no. 2, pp. 225-233, 2008.
M. Anson, J. Marchese, E. Garis, N. Ochoa, and C. Pagliero, “ABS copolymer-activated carbon mixed matrix membranes for CO2/CH4 seperation,” Journal of Membrane Science, vol. 243, pp. 19-28, 2004.
Y. G. Guo, Y. Zhao, and Y. J. Guo, “Removal of ammonia-nitrogen in wastewater by chitosan-coated micro-mesoporous zeolite,” Chinese Journal of Environmental Engineering, vol. 9, pp. 2067-2072, 2015.
A. Teimouri, S. G. Nasab, and N. Vahdatpoor, “Chitosan/ Zeolite Y /Nano ZrO2 nanocomposite as an adsorbent for the removal of nitrate from the aqueous solution,” International Journal of Biological Macromolecules, vol. 93, pp. 254-266, 2016.
M. Q. Sun, Y. N. Gao, and L. T. Zhou, “Study on optimization of modified chitosan for removal of nitrate by response surface methodology,” Environmental Engineering, vol. 36, pp. 33-37, 2018.
C. Hagyard, T. Cummings, and A. Martin, “Effect of ammonia exposure on subsequent rancid flavor development in lamp,” Journal of Muscle Food, vol. 4, no. 3, pp. 254-251, 1993.
A. Faber, Z, Jarosz, and T. Zylowski, “Verification of the possibilities to reduce ammonia emission for various slurry application practice in Poland,” Problems of World Agriculture, vol, 19, pp. 31-40, 2019.
A. Sapek, “Emission of ammonia from agriculture in Poland,” Zagadnienia Ekonomiki Rolnej, vol. 2, pp. 114-123, 2013.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












