Recovery of pure silver from spent silver electroplating solutions via electrochemical process and zinc cementation
DOI:
https://doi.org/10.55713/jmmm.v33i1.1576Keywords:
spent silver electroplating solution, electrowinning, electrorefining, recovery, purityAbstract
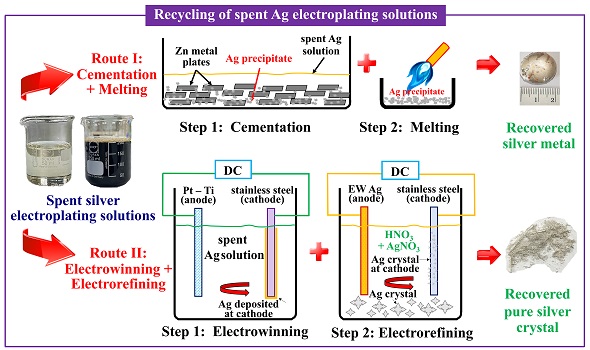
Recycling of spent silver electroplating solutions has been investigated via electrowinning and electrorefining in comparison to zinc cementation technique in this research. Two different compositions of transparent and dark waste solutions were used having the remaining silver contents of 17.71 g⸳L-1 and 33.36 g⸳L-1 respectively. The waste solutions were used as an electrolyte in the first step of electrowinning at low current density of 0.015 A⸳cm-2. It was found that increasing electrowinning time from 4 h to 24 h increased the recovery of silver cathode for both types of waste solutions. The optimum electrowinning time was higher than 8 h, giving the recovery of higher than 97.5% and 98.5% purity for 24 h electrowinning. Through the subsequent electrorefining, the electrowon silver cathode was set as the anode, while HNO3 + AgNO3 electrolyte containing high silver content of 120 g Ag/L was used. By controlling the potential at 0.8 V, silver crystal of high purity > 99.9% was obtained. The highest recovery was 99.11% when using silver cathode obtained from electrowinning of the transparent waste solution. Zinc cementation however led to loss of silver in the precipitate form on the zinc metal surface, giving only 86.16% recovery.
Downloads
References
ASTM B700-20: Standard specification for electrodeposited coatings of silver for engineering use.
M. Schlesinger, Chapter 5: Electroless and electrodeposition of silver, Modern Electroplating, John Wiley & Sons, Inc., 2010.
M. B. Mooiman, L. Simpson, Refining of Gold- and Silver-Bearing Doré in Gold Ore Processing, Gold Ore Processing, 2016.
N. G. K. Ramesh Bapu, C. Eagammai, S. Jayakrishnan, “Electrolytic recovery of silver from low concentrated silver cyanide spent plating solution” Transaction of the Institute of Metal Finishing, vol. 86, pp. 66-72, 2008.
J. R. Parga, R. Y. Wan, and J. D. Miller, “Zinc-dust cementation of silver from alkaline cyanide solutions analysis of Merrill-Crowe plant data,” Minerals and Metallurgical Processing, vol. 5, pp.170-176, 1988.
V. Väzquez, J. R Parga Torres, J. L. Valenzuela, G. Figueroa, A. Valenzuela, and G. T. Munive, “Recovery of silver from cyanide solutions using electrochemical process like alternative for Merrill – Crowe Process,” Materials Science and Applications, vol. 5, pp. 863-870, 2014.
O. N. Kononova, A. G. Kholmogorov, N. V. Danilenko, N. G. Goryaeva, K. A. Shatnykh, and S. V. Kachin, “Recovery of silver from thiosulfate and thiocyanate leach solutions by adsorption on anion exchange resins and activated carbon,” Hydrometallurgy, vol. 88, pp. 189-195, 2007.
F. Xie, D. Lu, H. Yang, and D. Dreisinger, “Solvent extraction of silver and gold from alkaline cyanide solution with LIX 7950,” Mineral Processing and Extractive Metallurgy Review, vol. 35, pp. 229-238, 2014.
A. K. Maurell-Lopez, B. Friedrich, W. Koch, “Challenges in the electrolytic refining of silver—influencing the Co-deposition through parameter control,” Rare Metal Technology, The Minerals, Metals & Materials Series. Springer, Cham, 2017.
O. Bayat, H. Vapur, F. Akyol, and C. Poole, “Effects of oxidising agents on dissolution of Gumuskoy silver ore in cyanide solution,” Minerals Engineering, vol. 16, pp. 395-398, 2003.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












