Tin oxide nanoparticle extraction from waste solder dross via low-temperature hydrometallurgical process
DOI:
https://doi.org/10.55713/jmmm.v35i2.2185Keywords:
SnO2, Nanoparticles, Tin oxide nanoparticles, Solder dross, HydrometallurgyAbstract
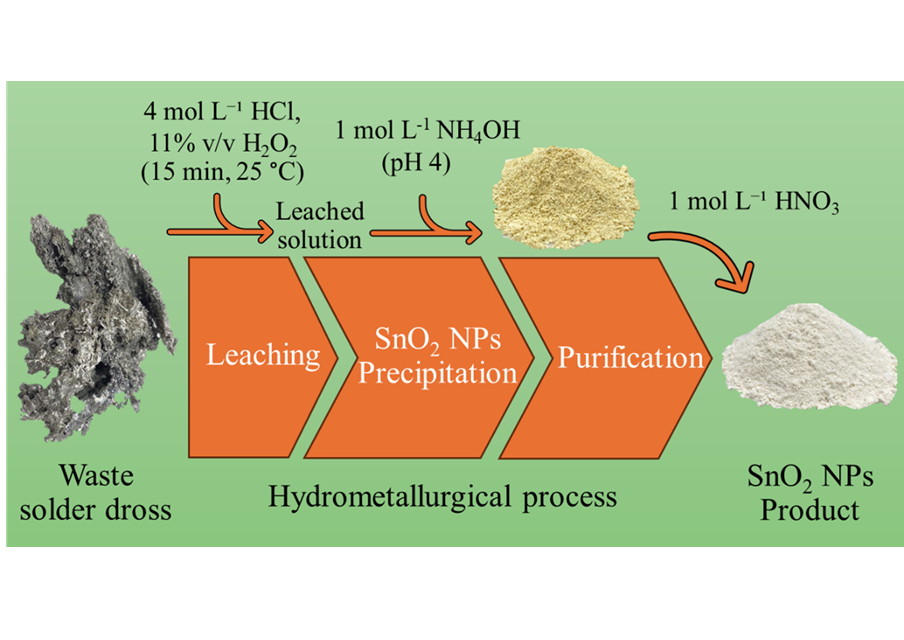
This study proposes a hydrometallurgical approach to valorising industrial waste by producing SnO2 nanoparticles (NPs) from waste solder dross obtained by skimming off of tin solder baths from electronic dipping processes. The process involves leaching the dross with aqueous hydrochloric acid solution and hydrogen peroxide, followed by precipitation with ammonium hydroxide and purification with aqueous nitric acid solution. The optimised leaching conditions were determined to be 4 mol∙ L‒1 HCl, 11% v/v H2O2, 25℃, and a leaching time of 15 min. SnO2 NPs were successfully precipitated at pH 4 using 25% v/v NH4OH and purified with 1 mol∙ L‒1 HNO3. The whole process was able to recover nearly 100% of tin from waste solder dross as SnO2 NPs, with a high purity of 99.5% and a particle size distribution of ca. 2 nm to 7 nm. The overall material balance and the simple cost of the process were evaluated to provide an idea of the economic viability and the possibility of scaling up the method detailed in this work.
Downloads
References
N. Kumar, and A. Maurya, "Development of lead free solder for electronic components based on thermal analysis," Materials Today: Proceedings, vol. 62, pp. 2163-2167, 2022.. DOI: https://doi.org/10.1016/j.matpr.2022.03.358
P. D. Sonawane, V. K. Bupesh Raja, K. Palanikumar, E. A. Kumar, N. Aditya, and V. Rohit, "Effects of gallium, phosphorus and nickel addition in lead-free solders: A review," Materials Today: Proceedings, vol. 46, pp. 3578-3581, 2021. DOI: https://doi.org/10.1016/j.matpr.2021.01.335
S. Jayesh, and J. Elias, "Experimental investigation on the effect of Ag addition on ternary lead free solder alloy –Sn–0.5Cu–3Bi," Metals and Materials International, vol. 26, no. 1, pp. 107-114, 2020. DOI: https://doi.org/10.1007/s12540-019-00305-3
S. Cheng, C.-M. Huang, and M. Pecht, "A review of lead-free solders for electronics applications," Microelectronics Reliability, vol. 75, pp. 77-95, 2017. DOI: https://doi.org/10.1016/j.microrel.2017.06.016
A. K. Gain, and L. Zhang, "Growth mechanism of intermetallic compound and mechanical properties of nickel (Ni) nanoparticle doped low melting temperature tin–bismuth (Sn–Bi) solder," Journal of Materials Science: Materials in Electronics, vol. 27, no. 1, pp. 781-794, 2016. DOI: https://doi.org/10.1007/s10854-015-3817-2
M. Sarkar, F. Gulshan, A. R. M. H. Rashid, and M. Hasanuzzaman, "A review of TiO2-nanoparticle reinforced lead-free solder composites used in electronic components soldering," in Encyclopedia of Materials: Electronics, A. S. M. A. Haseeb Ed. Oxford: Academic Press, 2023, pp. 456-463 DOI: https://doi.org/10.1016/B978-0-12-819728-8.00002-4
H. M. Henao, C. Masuda, and K. Nogita, "Metallic tin recovery from wave solder dross," International Journal of Mineral Processing, vol. 137, pp. 98-105, 2015. DOI: https://doi.org/10.1016/j.minpro.2015.02.006
I. García-Díaz, F. J. Alguacil, and F. A. López, "Tin and silver recovery from wave soldering dross," Waste Management & Research, vol. 36, no. 12, pp. 1201-1209, 2018. DOI: https://doi.org/10.1177/0734242X18798700
Z. Harangi, "Extraction of tin from oxidised soldering dross by carbothermic reduction and acid leaching," Materials Science and Engineering, vol. 39, 2014.
S.-h. Lee, K. Yoo, M. K. Jha, and J.-c. Lee, "Separation of Sn from waste Pb-free Sn–Ag–Cu solder in hydrochloric acid solution with ferric chloride," Hydrometallurgy, vol. 157, pp. 184-187, 2015. DOI: https://doi.org/10.1016/j.hydromet.2015.08.016
K. Yoo, J-C. Lee, K-S Lee, B-S. Kim, M-S. Kim, and B. D. Pandey, "Recovery of Sn, Ag and Cu from waste Pb-free solder using nitric acid leaching," Materials Transactions, vol. 53, no. 12, pp. 2175-2180, 2012. DOI: https://doi.org/10.2320/matertrans.M2012268
S. Kim, J.-c. Lee, K.-s. Lee, K. Yoo, and R. D. Alorro, "Separation of tin, silver and copper from waste pb-free solder using hydro-chloric acid leaching with hydrogen peroxide," Materials Transactions, vol. 55, no. 12, pp. 1885-1889, 2014. DOI: https://doi.org/10.2320/matertrans.M2014289
M. A. Dheyab, A. A. Aziz, M. S. Jameel, and N. Oladzadabbasabadi, "Recent advances in synthesis, modification, and potential application of tin oxide nanoparticles," Surfaces and Interfaces, vol. 28, p. 101677, 2022. DOI: https://doi.org/10.1016/j.surfin.2021.101677
S. Arya, M. Riyas, A. Sharma, B. Singh, Prerna, P. Bandhoria, S. Khan, and V Bharti, "Electrochemical detection of ammonia solution using tin oxide nanoparticles synthesised via sol–gel route," Applied Physics A, vol. 124, no. 8, p. 538, 2018. DOI: https://doi.org/10.1007/s00339-018-1968-8
S. R. Mishra, and M. Ahmaruzzaman, "Tin oxide based nano-structured materials: synthesis and potential applications," Nanoscale, vol. 14, no. 5, pp. 1566-1605, 2022. DOI: https://doi.org/10.1039/D1NR07040A
P. Liu, and V. Sivakov, "Tin/Tin oxide nanostructures: Formation, application, and atomic and electronic structure peculiarities," Nanomaterials, vol. 13, no. 17, p. 2391, 2023. DOI: https://doi.org/10.3390/nano13172391
J. Kharbanda, and R. Priya, "Synthesis and applications of tin oxide nanoparticles: An overview," Materials Today: Proceedings, vol. 68, pp. 916-921, 2022. DOI: https://doi.org/10.1016/j.matpr.2022.07.131
J. Mayandi, M. Marikkannan, V. Ragavendran, and P. Jayabal, "Hydrothermally synthesised Sb and Zn doped SnO2 nano-particles," Journal of Nanoscience and Nanotechnology, vol. 2, pp. 707-710, 2014.
O. A. Fouad, G. Glaspell, and M. S. EL-Shall, "Structural, optical and gas sensing properties of ZnO, SnO2 and ZTO nanostructures," Nano, vol. 05, no. 04, pp. 185-194, 2010. DOI: https://doi.org/10.1142/S1793292010002098
M. Kandasamy, A. Seetharaman, D. Sivasubramanian, A. Nithya, K. Jothivnkatachalam, N. Maheswari, M. Gopalan, S. Dillibabu, and A. Eftekhari, "Ni-doped SnO2 nanoparticles for sensing and photocatalysis," ACS Applied Nano Materials, vol. 1, no. 10, pp. 5823-5836, 2018. DOI: https://doi.org/10.1021/acsanm.8b01473
T. Entradas, J. F. Cabrita, S. Dalui, M. R. Nunes, O. C. Monteiro, and A. J. Silvestre, "Synthesis of sub-5 nm Co-doped SnO2 nanoparticles and their structural, microstructural, optical and photocatalytic properties," Materials Chemistry and Physics, vol. 147, no. 3, pp. 563-571, 2014. DOI: https://doi.org/10.1016/j.matchemphys.2014.05.032
M. Madhumala, D. Madhavi, T. Sankarshana, and S. Sridhar, "Recovery of hydrochloric acid and glycerol from aqueous solutions in chloralkali and chemical process industries by membrane distillation technique," Journal of the Taiwan Institute of Chemical Engineers, vol. 45, no. 4, pp. 1249-1259, 2014. DOI: https://doi.org/10.1016/j.jtice.2014.02.010
C. McKinley, and A. Ghahreman, "Hydrochloric acid regeneration in hydrometallurgical processes: a review," Mineral Processing and Extractive Metallurgy, vol. 127, no. 3, pp. 157-168, 2018. DOI: https://doi.org/10.1080/03719553.2017.1330839
S.-k. Kim, J.-c. Lee, and K. Yoo, "Leaching of tin from waste Pb-free solder in hydrochloric acid solution with stannic chloride," Hydrometallurgy, vol. 165, pp. 143-147, 2016. DOI: https://doi.org/10.1016/j.hydromet.2015.09.018
S. Li, J. Ma, F. Xu, L. Wei, and D. He, "Fundamental principles and environmental applications of electrochemical hydrogen peroxide production: A review," Chemical Engineering Journal, vol. 452, p. 139371, 2023. DOI: https://doi.org/10.1016/j.cej.2022.139371
S. Ghosh and S. Roy, "Effect of ageing on Sn6O4(OH)4 in aqueous medium—simultaneous production of SnO and SnO2 nanoparticles at room temperature," Journal of Sol-Gel Science and Technology, vol. 81, no. 3, pp. 769-773, 2017. DOI: https://doi.org/10.1007/s10971-016-4251-5
J. Geurts, S. Rau, W. Richter, and F. J. Schmitte, "SnO films and their oxidation to SnO2: Raman scattering, IR reflectivity and X-ray diffraction studies," Thin Solid Films, vol. 121, no. 3, pp. 217-225, 1984. DOI: https://doi.org/10.1016/0040-6090(84)90303-1
Z. T. Ichlas, R. A. Rustandi, and M. Z. Mubarok, "Selective nitric acid leaching for recycling of lead-bearing solder dross," Journal of Cleaner Production, vol. 264, p. 121675, 2020. DOI: https://doi.org/10.1016/j.jclepro.2020.121675
V. P. Sankara Narayanan, S. G. Kathirason, P. Elango, R. Subramanian, S. R, and A. Gurusamy, "Emilia sonchifolia leaf extract-mediated green synthesis, characterisation, in vitro biological activities, photocatalytic degradation and in vivo Danio rerio embryo toxicity of copper nanoparticles," RSC Advances, vol. 13, no. 24, pp. 16724-16740, 2023. DOI: https://doi.org/10.1039/D3RA00454F
M. Karmaoui, A. B. J. Sobrido, P. McMillan, A. E. Aliev, R. C. Pullar, J. A. Labrincha, and D. M. Tobaldi, "One-step synthesis, structure, and band gap properties of SnO2 nanoparticles made by a low temperature nonaqueous sol-gel technique," (in eng), ACS Omega, vol. 3, no. 10, pp. 13227-13238, 2018. DOI: https://doi.org/10.1021/acsomega.8b02122
A. Y. Fosu, D. Bartier, F. Diot, and N. Kanari, "Insight into the extractive metallurgy of tin from cassiterite," Materials, vol. 17, no. 13, p. 3312, 2024,. DOI: https://doi.org/10.3390/ma17133312
Z. Su, Y. Zhang, B. Liu, M. Lu, G. Li, and T. Jiang, "Extraction and separation of tin from tin-bearing secondary resources: A review," JOM, vol. 69, no. 11, pp. 2364-2372, 2017. DOI: https://doi.org/10.1007/s11837-017-2509-1
S. Khumkoa, N. Ma-ud, T. Patcharawit, and T. Yingnakorn, "Tin, silver, and copper sulfate compound extraction from lead-free solder dross by reduction with petroleum coke, electrorefining, cementation, and crystallisation process," Journal of Metals, Materials and Minerals, vol. 34, no. 4, p. 2007, 2024. DOI: https://doi.org/10.55713/jmmm.v34i4.2007
N. Ma-ud, S. Khumkoa, P. Buahombura, W. Piyawit, T. Patcharawit, S. Thongnak, and T. Yingnakorn, "Study on recycling of galvanic sludge containing copper for pure copper production," Journal of Material Sciences & Engineering, vol. 8, no. 2169-0022, 2019.
T. Patcharawit, C. Kansomket, N. Wongnaree, W. Kritsrikan, T. Yingnakorn, and S. Khumkoa, "Hybrid recovery of copper and silver from PV ribbon and Ag finger of EOL solar panels," Energy and Power Engineering, vol. 16, no. 6, pp. 89-99, 2022.
Downloads
Published
How to Cite
License
Copyright (c) 2025 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












