Corrosion behavior of YSZ and YSZ/NiCo coatings on inconel 625 exposed alkali chlorides
DOI:
https://doi.org/10.55713/jmmm.v34i2.1879Keywords:
YSZ, Electrophoretic Deposition, alkali chlorides, hot salt corrosionAbstract
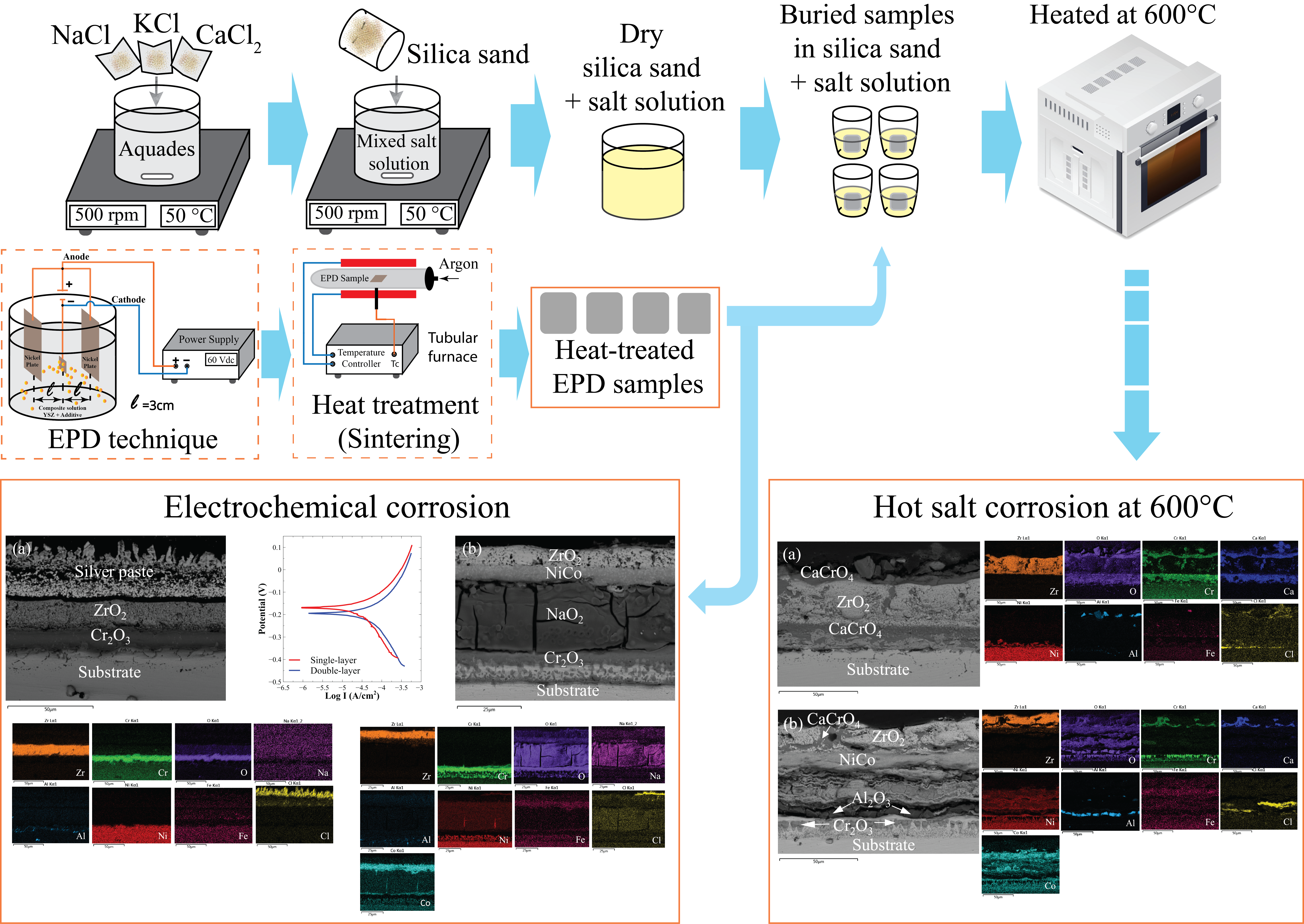
Alkali chloride attack on boiler pipe walls is considered the main problem of corrosion in the waste-to-energy (WTE) industry, even though uses superalloy. Electrophoretic deposited (EPD) yttria-stabilized zirconia (YSZ) coating is carried out to protect the Inconel 625 substrate. YSZ is deposited directly both on the Inconel 625 substrate and NiCo-Inconel 625. Corrosion resistance was conducted using the 3.5% NaCl electrochemical test and the hot salt corrosion test at 600°C in alkaline salt media such as NaCl, KCl, and CaCl2. The potentiodynamic polarization curve shows that the YSZ coating deposited on the substrate (single-layer) has a corrosion rate of 0.065 mm∙y‒1, lower than that deposited on NiCo coating (double-layer). The double-layer, NiO2 is formed in the NiCo layer due to the NaCl solution being trapped. Meanwhile, in hot salt corrosion at 600°C, CaCrO4 is formed as a protective oxide layer. Furthermore, in the double-layer, an imperfect oxide layer is formed causing spallation and coating failure. The corrosion rate for single-layer hot salt corrosion for 40 h is 0.310 mm∙y‒1. As a result, the corrosion resistance of the single-layer is increased by the presence of the Cr2O3 oxide layer formed during sintering.
Downloads
References
H. Izzuddin, S. Hayashi, S. Yoneda, T. Kogin, E. Ishikawa, and M. Noguchi, “Effect of Mo on corrosion behavior of Ni20Cr–xMo alloys in air with NaCl–KCl–CaCl2 vapor at 570°C,” Materials and Corrosion, vol. 71, no. 9, pp. 1488-1499, 2020. DOI: https://doi.org/10.1002/maco.201911469
J. Liu, D. Dyson, and E. Asselin, “Long-term hot corrosion behavior of boiler tube alloys in waste-to-energy plants,” Oxidation of Metals, vol. 86, no. 1-2, pp. 135-149, 2016. DOI: https://doi.org/10.1007/s11085-016-9627-y
G. Sorell, “The role of chlorine in high temperature corrosion in waste-to-energy plants,” Materials at High Temperatures, vol. 14, no. 3, pp. 207-220, 1997. DOI: https://doi.org/10.1080/09603409.1997.11689546
N. Otsuka, “A thermodynamic approach on vapor-condensation of corrosive salts from flue gas on boiler tubes in waste incinerators,” Corrosion Science, vol. 50, no. 6, pp. 1627-1636, 2008. DOI: https://doi.org/10.1016/j.corsci.2008.02.004
S.-H. Lee, N. J. Themelis, and M. J. Castaldi, “High-temperature corrosion in waste-to-energy boilers,” Journal of Thermal Spray Technology, vol. 16, no. 1, pp. 104-110, 2007. DOI: https://doi.org/10.1007/s11666-006-9005-4
M. Sánchez Pastén, and M. Spiegel, “High temperature corrosion of metallic materials in simulated waste incineration environments at 300–600°C,” Materials and Corrosion, vol. 57, no. 2, pp. 192-195, 2006. DOI: https://doi.org/10.1002/maco.200503909
L. B. Chen, “Yttria-stabilized zirconia thermal barrier coatings - A review,” Surface Review and Letters, vol. 13, no. 05, pp. 535-544, 2006. DOI: https://doi.org/10.1142/S0218625X06008670
X. Q. Cao, R. Vassen, and D. Stoever, “Ceramic materials for thermal barrier coatings,” Journal of the European Ceramic Society, vol. 24, no. 1, pp. 1-10, 2004. DOI: https://doi.org/10.1016/S0955-2219(03)00129-8
N. P. Padture, M. Gell, and E. H. Jordan, “Thermal barrier coatings for gas-turbine engine applications,” Science, vol. 296, no. 5566, pp. 280-284, 2002. DOI: https://doi.org/10.1126/science.1068609
M. Bai, F. Guo, and P. Xiao, “Fabrication of thick YSZ thermal barrier coatings using electrophoretic deposition,” Ceramics International, vol. 40, no. 10, pp. 16611-16616, 2014. DOI: https://doi.org/10.1016/j.ceramint.2014.08.021
K. Sharifi and M. Ghorbani, “Corrosion behaviour of Ni–Co alloy coatings at Kish Island (Marine) atmosphere,” Bulletin of Materials Science, vol. 37, no. 3, pp. 713-719, 2014. DOI: https://doi.org/10.1007/s12034-014-0668-z
E. Sugiarti, R. D. Desiati, K. A. Zaini, K. Hartanto, and N. Prastomo, “Effect of Co concentration on hardness of NiCo coating layer synthesized by electroplating method,” Journal of Physics: Conference Series, vol. 1191, p. 012062, 2019. DOI: https://doi.org/10.1088/1742-6596/1191/1/012062
R. D. Desiati, A. Anawati, and E. Sugiarti, “Two-step sintering improved compaction of electrophoretic-deposited YSZ coatings,” Journal of Materials Engineering and Performance, vol. 31, no. 12, pp. 9888-9899, 2022. DOI: https://doi.org/10.1007/s11665-022-07004-y
T. J. Lucas, N. C. Lawson, G. M. Janowski, and J. O. Burgess, “Effect of grain size on the monoclinic transformation, hardness, roughness, and modulus of aged partially stabilized zirconia,” Dental Materials, vol. 31, no. 12, pp. 1487-1492, 2015. DOI: https://doi.org/10.1016/j.dental.2015.09.014
S. E. Redfern, R. W. Grimes, and R. D. Rawlings, “The hydroxylation of t-ZrO2 surfaces,” Journal of Materials Chemistry, vol. 11, no. 2, pp. 449-455, 2001. DOI: https://doi.org/10.1039/b007789p
B. Butz, R. Schneider, D. Gerthsen, M. Schowalter, and A. Rosenauer, “Decomposition of 8.5 mol.% Y2O3-doped zirconia and its contribution to the degradation of ionic conductivity,” Acta Materialia, vol. 57, no. 18, pp. 5480-5490, 2009. DOI: https://doi.org/10.1016/j.actamat.2009.07.045
H. Hayashi, T. Saitou, N. Maruyama, H. Inaba, K. Kawamura, and M. Mori, “Thermal expansion coefficient of yttria stabilized zirconia for various yttria contents,” Solid State Ionics, vol. 176, no. 5-6, pp. 613-619, 2005. DOI: https://doi.org/10.1016/j.ssi.2004.08.021
M. Yashima, K. Ohtake, H. Arashi, M. Kakihana, and M. Yoshimura, “Determination of cubic‐tetragonal phase boundary in Zr1−X YXO 2−X/2 solid solutions by Raman spectroscopy,” Journal of Applied Physics, vol. 74, no. 12, pp. 7603-7605, 1993. DOI: https://doi.org/10.1063/1.354989
P. Amrollahi, J. S. Krasinski, R. Vaidyanathan, L. Tayebi, and D. Vashaee, “Electrophoretic deposition (EPD): Fundamentals and applications from nano- to microscale structures,” in Handbook of Nanoelectrochemistry, M. Aliofkhazraei and A. S. H. Makhlouf, Eds. Cham: Springer International Publishing, 2016, pp. 561-591. DOI: https://doi.org/10.1007/978-3-319-15266-0_7
R. D. Desiati, A. Anawati, and E. Sugiarti, “Microstructural and mechanical characteristic of ceramic composite coating developed by electrophoretic deposition,” IOP Conference Series: Materials Science Engineering, vol. 1098, no. 6, p. 062073, 2021. DOI: https://doi.org/10.1088/1757-899X/1098/6/062073
H. Negishi, K. Yamaji, N. Sakai, T. Horita, H. Yanagishita, and H. Yokokawa, “Electrophoretic deposition of YSZ powders for solid oxide fuel cells,” Journal of Materials Science, vol. 39, no. 3, pp. 833-838, 2004. DOI: https://doi.org/10.1023/B:JMSC.0000012911.86185.13
“Data Sheet Inconel 625.” www.specialmetals.com, 13-Aug-2013.
“HSC Chemistry Software.” Outotec (Finland) Oy, 2019. DOI: https://doi.org/10.1016/S1365-6937(19)30217-5
N. Hiraide, T. Muneno, and H. Kajimura, “Reaction of Cr and Cr oxide with NaCl at elevated temperature,” Zairyo-to-Kankyo, vol. 58, no. 10, pp. 348-355, 2009. DOI: https://doi.org/10.3323/jcorr.58.348
S. Esmailzadeh, M. Aliofkhazraei, and H. Sarlak, “Interpretation of Cyclic potentiodynamic polarization test results for study of corrosion behavior of metals: A Review,” Protection of Metals and Physical Chemistry of Surfaces, vol. 54, no. 5, pp. 976-989, 2018. DOI: https://doi.org/10.1134/S207020511805026X
E. McCafferty, Introduction to Corrosion Science. New York, NY: Springer New York, 2010. DOI: https://doi.org/10.1007/978-1-4419-0455-3
S. Karlsson, J. Pettersson, L.-G. Johansson, and J.-E. Svensson, “Alkali induced high temperature corrosion of stainless steel: The influence of NaCl, KCl and CaCl2,” Oxidation of Metals, vol. 78, no. 1-2, pp. 83-102, 2012. DOI: https://doi.org/10.1007/s11085-012-9293-7
H. Shahbazi, H. Vakilifard, R. B. Nair, A. C. Liberati, R. S. Lima, P. Stoyanov, and C. Moreau, “High entropy alloy bond coats for thermal barrier coatings: A review,” Journal of Thermal Spray Technology, vol. 33, no. 10, 2023. DOI: https://doi.org/10.31399/asm.cp.itsc2023p0659
Centre international de recherche sur le cancer, Ed., A review of human carcinogens. Lyon: International agency for research on cancer, 2012.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












