Multi-responsive rotaxane with tunable fluorescence under azobenzene-based benzoxazine structure
DOI:
https://doi.org/10.55713/jmmm.v32i3.1269คำสำคัญ:
Rotaxane, Benzoxazine, Light responsiveness, Supramoleculeบทคัดย่อ
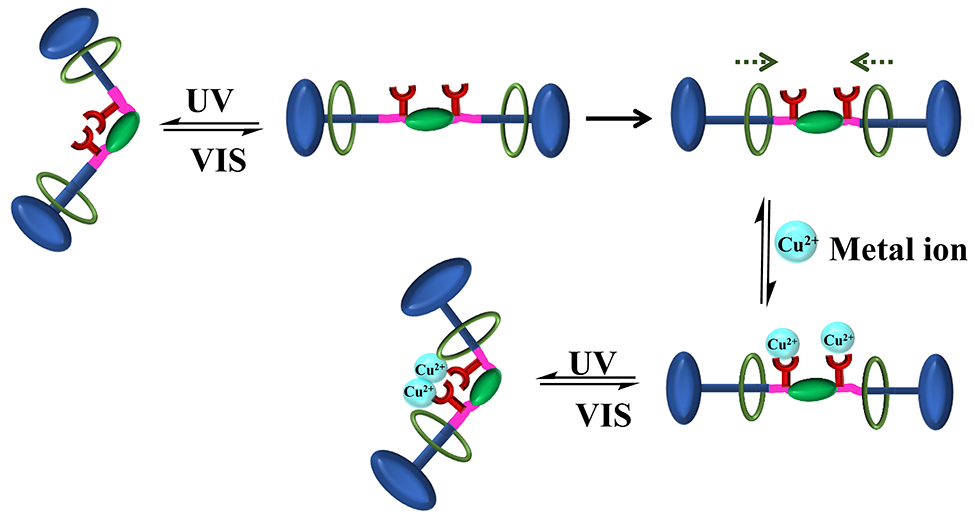
Rotaxanes are known for the mechanically interlocked molecules for decades. The present work demonstrates a method to prepare multi-responsive rotaxane by conjugating with a multi-responsive supramolecule. Benzoxazine dimers, N, N’-bis(3,5-dimethyl-2-hydroxybenzyl) methylamine derivatives, are good models because their simple chemistry. An azobenzene containing benzoxazine with remaining hydroxyl group for further conjugation with rotaxane was designed. The ring opening of rotaxane using fluorescent phenol provides benzoxazine dimer with metal ion responsive and fluorescent properties. Based on this concept, light responsive benzoxazine conjugated with rotaxane system shows light, metal ion and rotaxane shuttling responsiveness which can be followed by fluorescent signals. The present work shows simple way to develop rotaxanes with multi-responsive functions using supramolecular chemistry of benzoxazine dimer prepared from light responsive phenol.
Downloads
เอกสารอ้างอิง
X. Chi, X. Ji, D. Xia, and F. Huang, "A Dual-Responsive Supra-Amphiphilic Polypseudorotaxane Constructed from a Water-Soluble Pillar[7]arene and an Azobenzene-Containing Random Copolymer," Journal of the American Chemical Society, vol. 137, no. 4, pp. 1440-1443, 2015. DOI: https://doi.org/10.1021/ja512978n
X. Chi, G. Yu, L. Shao, J. Chen, and F. Huang, "A Dual-Thermoresponsive Gemini-Type Supra-amphiphilic Macromolecular [3]Pseudorotaxane Based on Pillar[10]arene/Paraquat Cooperative Complexation," Journal of the American Chemical Society, vol. 138, no. 9, pp. 3168-3174, 2016. DOI: https://doi.org/10.1021/jacs.5b13173
J. Zhou, G. Yu, and F. Huang, "Supramolecular chemotherapy based on host–guest molecular recognition: a novel strategy in the battle against cancer with a bright future," Chemical Society Reviews, vol. 46, no. 22, pp. 7021-7053, 2017. DOI: https://doi.org/10.1039/C6CS00898D
G. S. Kumar and D. C. Neckers, "Photochemistry of azobenzene-containing polymers," Chemical Reviews (Washington, DC, United States), vol. 89, no. 8, pp. 1915-1925, 1989. DOI: https://doi.org/10.1021/cr00098a012
M.-M. Russew and S. Hecht, "Photoswitches: From Molecules to Materials," Advanced Materials (Weinheim, Germany), vol. 22, no. 31, pp. 3348-3360, 2010. DOI: https://doi.org/10.1002/adma.200904102
Y. Yu, M. Nakano, and T. Ikeda, "Photomechanics: Directed bending of a polymer film by light," Nature, vol. 425, no. 6954, pp. 145-145, 2003. DOI: https://doi.org/10.1038/425145a
S. Lee, Y. You, K. Ohkubo, S. Fukuzumi, and W. Nam, "Mechanism and Fluorescence Application of Electrochromism in Photochromic Dithienylcyclopentene," Organic Letters, vol. 14, no. 9, pp. 2238-2241, 2012. DOI: https://doi.org/10.1021/ol300604n
G. K. Such, R. A. Evans, and T. P. Davis, "Control of Photochromism through Local Environment Effects Using Living Radical Polymerization (ATRP)," Macromolecules, vol. 37, no. 26, pp. 9664-9666, 2004. DOI: https://doi.org/10.1021/ma048627f
A. B. Othman et al., "Calix[4]arene-Based, Hg2+-Induced Intramolecular Fluorescence Resonance Energy Transfer Chemosensor," Journal of Organic Chemistry, vol. 72, no. 20, pp. 7634-7640, 2007. DOI: https://doi.org/10.1021/jo071226o
S. Phongtamrug, K. Tashiro, M. Miyata, and S. Chirachanchai, "Supramolecular Structure of N,N-Bis(2-hydroxybenzyl)alkylamine: Flexible Molecular Assembly Framework for Host without Guest and Host with Guest," Journal of Physical Chemistry B, vol. 110, no. 42, pp. 21365-21370, 2006. DOI: https://doi.org/10.1021/jp061778v
S. Phongtamrug, B. Pulpoka, and S. Chirachanchai, "Inclusion Compounds Formed from N,N-bis(2-hydroxybenzyl)alkylamine Derivatives and Transition Metal Ions via Molecular Assembly," Supramolecular Chemistry, vol. 16, no. 4, pp. 269-278, 2004. DOI: https://doi.org/10.1080/1061027042000204029
S. Phongtamrug, S. Chirachanchai, and K. Tashiro, "Supramolecular Structure of N,N-Bis(2-hydroxy-benzyl)alkylamine: From Hydrogen Bond Assembly to Coordination Network in Guest Acceptance," Macromolecular Symposia, vol. 242, no. 1, pp. 40-48, 2006. DOI: https://doi.org/10.1002/masy.200651007
D. Aoki, S. Uchida, and T. Takata, "Synthesis and characterization of a mechanically linked transformable polymer," Polymer Journal (Tokyo, Japan), vol. 46, no. 9, pp. 546-552, 2014. DOI: https://doi.org/10.1038/pj.2014.22
D. Aoki, S. Uchida, and T. Takata, "Star/Linear Polymer Topology Transformation Facilitated by Mechanical Linking of Polymer Chains," Angewandte Chemie, International Edition, pp. 6770–6774, 2015. DOI: https://doi.org/10.1002/anie.201500578
T. Ogawa, K. Nakazono, D. Aoki, S. Uchida, and T. Takata, "Effective Approach to Cyclic Polymer from Linear Polymer: Synthesis and Transformation of Macromolecular [1]Rotaxane," ACS Macro Letters, vol. 4, no. 4, pp. 343-347, 2015. DOI: https://doi.org/10.1021/acsmacrolett.5b00067
T. Ogawa, N. Usuki, K. Nakazono, Y. Koyama, and T. Takata, "Linear-cyclic polymer structural transformation and its reversible control using a rational rotaxane strategy," Chemical Communications (Cambridge, United Kingdom), vol. 51, no. 26, pp. 5606-5609, 2015. DOI: https://doi.org/10.1039/C4CC08982K
H. W. Gibson et al., "Supramacromolecular self-assembly: Chain extension, star and block polymers via pseudorotaxane formation from well-defined end-functionalized polymers," Journal of Polymer Science Part A: Polymer Chemistry, vol. 47, no. 14, pp. 3518-3543, 2009. DOI: https://doi.org/10.1002/pola.23435
S.-M. Chan, F.-K. Tang, C.-S. Kwan, C.-Y. Lam, S. C. K. Hau, and K. C.-F. Leung, "Water-compatible fluorescent [2]rotaxanes for Au3+ detection and bioimaging," Materials Chemistry Frontiers, vol. 3, no. 11, pp. 2388-2396, 2019. DOI: https://doi.org/10.1039/C9QM00476A
M. Arunachalam and H. W. Gibson, "Recent developments in polypseudorotaxanes and polyrotaxanes," Progress in Polymer Science, vol. 39, no. 6, pp. 1043-1073, 2014. DOI: https://doi.org/10.1016/j.progpolymsci.2013.11.005
K. Iijima et al., "Stimuli-degradable cross-linked polymers synthesized by radical polymerization using a size-complementary [3]rotaxane cross-linker," Polymer Journal (Tokyo, Japan), vol. 46, no. 1, pp. 67-72, 2014. DOI: https://doi.org/10.1038/pj.2013.63
J. Sawada, D. Aoki, S. Uchida, H. Otsuka, and T. Takata, "Synthesis of Vinylic Macromolecular Rotaxane Cross-Linkers Endowing Network Polymers with Toughness," ACS Macro Letters, vol. 4, no. 5, pp. 598-601, 2015. DOI: https://doi.org/10.1021/acsmacrolett.5b00242
Y. Koyama, T. Matsumura, T. Yui, O. Ishitani, and T. Takata, "Fluorescence Control of Boron Enaminoketonate Using a Rotaxane Shuttle," Organic Letters, vol. 15, no. 18, pp. 4686-4689, 2013. DOI: https://doi.org/10.1021/ol401984j
Y. Abe, H. Okamura, S. Uchida, and T. Takata, "Synthesis of main chain-type liquid crystalline polyrotaxanes: influence of the wheel components and their mobility on liquid crystalline properties," Polymer Journal (Tokyo, Japan), vol. 46, no. 9, pp. 553-558, 2014. DOI: https://doi.org/10.1038/pj.2014.23
S. Suzuki, K. Matsuura, K. Nakazono, and T. Takata, "Effect of a side chain rotaxane structure on the helix-folding of poly(m-phenylene diethynylene)," Polymer Journal (Tokyo, Japan), vol. 46, no. 6, pp. 355-365, 2014. DOI: https://doi.org/10.1038/pj.2014.4
L. Zhu, X. Ma, F. Ji, Q. Wang, and H. Tian, "Effective Enhancement of Fluorescence Signals in Rotaxane-Doped Reversible Hydrosol–Gel Systems," Chemistry--A European Journal, vol. 13, no. 33, pp. 9216-9222, 2007. DOI: https://doi.org/10.1002/chem.200700860
A. S. D. Sandanayaka, H. Sasabe, T. Takata, and O. Ito, "Photoinduced electron transfer processes of fullerene rotaxanes containing various electron-donors," Journal of Photochemistry and Photobiology, C: Photochemistry Reviews, vol. 11, no. 2–3, pp. 73-92, 2010. DOI: https://doi.org/10.1016/j.jphotochemrev.2010.05.001
M. Denis, J. Pancholi, K. Jobe, M. Watkinson, and S. M. Goldup, "Chelating Rotaxane Ligands as Fluorescent Sensors for Metal Ions," Angewandte Chemie International Edition, vol. 57, no. 19, pp. 5310-5314, 2018. DOI: https://doi.org/10.1002/anie.201712931
T. Clifford, A. Abushamleh, and D. H. Busch, "Factors affecting the threading of axle molecules through macrocycles: Binding constants for semirotaxane formation," Proceedings of the National Academy of Sciences of the United States of America, vol. 99, no. 8, pp. 4830-4836, 2002. DOI: https://doi.org/10.1073/pnas.062639799
S. Yu, N. D. McClenaghan, and J.-L. Pozzo, "Photochromic rotaxanes and pseudorotaxanes," Photochemical & Photobiological Sciences, vol. 18, no. 9, pp. 2102-2111, 2019. DOI: https://doi.org/10.1039/C9PP00057G
ดาวน์โหลด
เผยแพร่แล้ว
วิธีการอ้างอิง
ฉบับ
บท
การอนุญาต
ลิขสิทธิ์ (c) 2022 วารสารโลหะ, วัสดุ และแร่

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












