Development of inexpensive device for methanol detection in hand sanitizer gel using manganese-doped zinc sulfide quantum dots modified by N-methylpolypyrrole
DOI:
https://doi.org/10.55713/jmmm.v33i1.1597Keywords:
Methanol detection, Manganese-doped zinc sulfide quantum dots, N-methyl polypyrrole, Digital-image colorimetry, Hand sanitizer gelAbstract
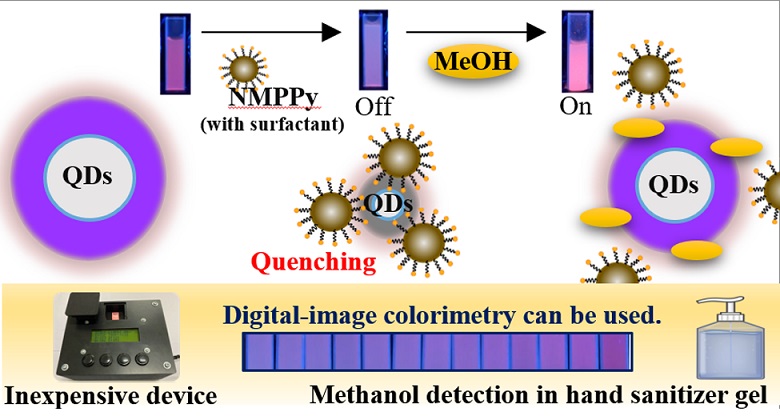
A method for methanol detection by an inexpensive device using a nanomaterial modified by N-methylpolypyrrole (NMPPY) has been developed. Manganese-doped zinc sulfide quantum dots (Mn/ZnS-QDs) were synthesized and then characterized by a fluorescence spectrophotometer to study their spectroscopic properties. Mn/ZnS-QDs were modified with NMPPy and studied by digital-image colorimetry to optimize conditions for methanol detection. A 2 mL of 3000 mg∙L–1 Mn/ZnS-QDs modified with 300 μL of 1000 mg∙L-1 of NMPPy was chosen to be a detecting reagent for methanol determination. Under the optimum conditions, the linear range was found to be 2%v/v to 50%v/v of methanol with R-square of 0.9434 and the sensitivity of 3.569 ´ 10–3 (%v/v)-1, whilst the limit of detection (LOD) was 19.5%v/v. The selectivity of this method was also studied with several solvents; it was proven selective for methanol. Furthermore, a prototype device with simple and inexpensive has been created. The analytical performances were studied; the linearity of methanol detection was found in the range of 20%v/v to 80%v/v with R-square of 0.9918 and the sensitivity of 3.38 ´ 10-3 (%v/v)-1. Finally, the newly developed device was applied to analyze samples of hand sanitizer gel by digital-image colorimetry with acceptable results.
Downloads
References
M. Svanberg, J. Ellis, J. Lundgren, and I. Landälv, "Renewable methanol as a fuel for the shipping industry," Renewable and Sustainable Energy Reviews, vol. 94, pp. 94:1217, 2018.
M. A. Kostic, and R. C. Dart, "Rethinking the toxic methanol level," Journal of Toxicology. Clinical Toxicology, vol. 41, pp. 793-800, 2003.
G. Leaf, and L. J. Zatman, "A study of the conditions under which methanol may exert a toxic hazard in industry," British Journal of Industrial Medicine, vol. 9, pp. 19-31, 1952.
S. Baloch, M. A. Baloch, T. Zheng, and X. Pei, "The coronavirus disease 2019 (COVID-19) Pandemic," The Tohoku Journal of Experimental Medicine, vol. 250, pp. 271-278, 2020.
G. C. Martin, G. Le Roux, D. Guindolet, E. Boulanger, D. Hasle, E. Morin, D. Vodovar, C. Vignal, E. Gabison, and A. Descatha, "Pediatric eye injuries by hydroalcoholic gel in the context of the coronavirus disease 2019 pandemic," JAMA Ophthalmology, vol. 139, pp. 348-351, 2021.
W. Sattle, H. Puhl, M. Hayn, G. M. Kostner, and H. Esterbauer, "Determination of fatty acids in the main lipoprotein classes by capillary gas chromatography: BF3/methanol transesterification of lyophilized samples instead of folch extraction gives higher yields," Analytical Biochemistry, vol. 198, pp. 184-190, 1991.
B. Tao, J. Zhang, S. Hui, X. Chen, and L. Wan, "An electro-chemical methanol sensor based on a Pd–Ni/SiNWs catalytic electrode," Electrochimica Acta, vol. 55, pp. 5019-5023, 2010.
B. R. Buchanan, and D. E. Honigs, "Detection of methanol in gasolines using near-infrared spectroscopy and an optical fiber," Applied Spectroscopy, vol. 41, pp. 1388-1392,1987.
M. Nemecek-Marshall, R. C. MacDonald, J. J. Franzen, C. L. Wojciechowski, and R. Fall, "Methanol emission from leaves (enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development), "Plant Physiology, vol.108, pp. 1359-1368, 1995.
N. G. Patel, P.D. Patel, and V. S. Vaishnav, "Indium tin oxide (ITO) thin film gas sensor for detection of methanol at room temperature," Sensors and Actuators B: Chemical, vol. 96, pp. 180-189, 2003.
M. Rana, and P. Chowdhury, "L-glutathione capped CdSeS/ ZnS quantum dot sensor for the detection of environmentally hazardous metal ions," Journal of Luminescence, vol. 206, pp. 105-112, 2019.
A. P. Alivisatos, "Semiconductor clusters, nanocrystals, and quantum dots," Science, vol. 271, pp. 933-937, 1996.
J. K. Jaiswal, and S. M. Simon, "Potentials and pitfalls of fluorescent quantum dots for biological imaging," Trends in Cell Biology, vol. 14, pp. 497-504, 2004.
W. Bian, J. Ma, W. Guo, D. Lu, M. Fan, Y. Wei, Y. Li, S. Shuang, and M. M. F. Choi, "Phosphorescence detection of L-ascorbic acid with surface-attached N-acetyl-L-cysteine and L-cysteine Mn doped ZnS quantum dots," Talanta, vol. 116, pp. 794-800, 2013.
F. Abbasi, A. Akbarinejad, and N. Alizadeh, "CdS QDs/N-methylpolypyrrole hybrids as fluorescent probe for ultrasensitive and selective detection of picric acid," Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, vol. 216, pp. 230-235, 2019.
F. Abbasi, and N. Alizadeh, "Highly selective detection of methanol in aqueous and ethanol medium based on hybrid ZnS:Mn2+quantum dots/N-methylpolypyrrole as a fluorescence switchable sensor," Food Chemistry, vol. 328, pp.127091, 2020.
A. C. Small, and J. H. Johnston, "Novel hybrid materials of cellulose fibres and doped ZnS nanocrystals," Current Applied Physics, vol. 8, pp. 512-515, 2008.
N. Alizadeh, and A. Akbarinejad, "Soluble fluorescent polymeric nanoparticles based on pyrrole derivatives: synthesis, characterization and their structure dependent sensing properties," Journal of Materials Chemistry C, vol. 3, pp. 9910-9920, 2015.
J. Hottechamps, T. Noblet, A. Brans, C. Humbert, and L. Dreesen, "How quantum dots aggregation enhances Förster resonant energy transfer," ChemPhysChem, vol. 21, pp. 853-862, 2020.
E. Sharon, R. Freeman, M. Riskin, N. Gil, Y. Tzfati ,and I. Willner, "Optical, electrical and surface plasmon resonance methods for detecting telomerase activity," Analytical Chemistry, vol. 82, pp. 8390-8397, 2010.
P. Mukoma, B. R. Jooste, and H. C. M. Vosloo, "A comparison of methanol permeability in chitosan and nafion 117 membranes at high to medium methanol concentrations," Journal of Membrane Science, vol. 243, pp. 293-299, 2004.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












