ZnO nanostructures synthesized by one-step sol-gel process using different zinc precursors
DOI:
https://doi.org/10.55713/jmmm.v34i3.1968คำสำคัญ:
ZnO nanostructures, sol-gel process, starting precursorบทคัดย่อ
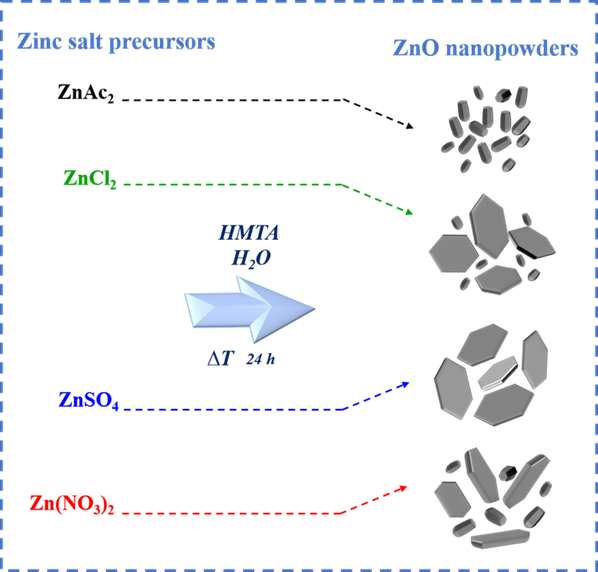
Zinc oxide (ZnO) nanopowders have been widely applied in electronics, optics and photocatalytic applications depending on their morphological structure. In the bottom-up process, it is conceived that the different zinc precursors may result in different formations of ZnO nanostructures with exceptional morphology. This work focuses on ZnO material synthesized via the facile sol-gel synthesis using different zinc slat precursors, including zinc acetate, zinc nitrate, zinc sulphate, and zinc chloride. All zinc salt precursors were incorporated with sodium hydroxide and hexamethylenetetramine (HMTA) under mild thermal energy with consistent conditions to investigate ZnO formation. The as-prepared samples appeared in white powders with different aggregation features. The crystalline phase, surface morphologies, and element mapping of all ZnO samples were analyzed using X-ray diffraction technique (XRD) and field emission scanning electron microscope (FE-SEM). The chemical bonding structure of ZnO powders was characterized by Fourier transform infrared spectroscopy (FTIR) and Raman spectroscopy. The specific surface area per volume of ZnO nanopowders obtained by different zinc salt precursors was analyzed by Brunauer-Emmett-Teller (BET) method. All ZnO samples obtained from various zinc salt precursors exhibited a high crystallinity of the wurtzite structure without other impurities. The structural properties of ZnO nanopowders demonstrated different sizes and structures with distinguished formation and aggregation depending on the zinc precursor basic strength being used.
Downloads
เอกสารอ้างอิง
A. N. U. Haq, A. Nadhman, I. Ullah, G. Mustafa, M. Yasinzai, and I. Khan, “Synthesis approaches of zinc oxide nanoparticles: the dilemma of ecotoxicity,” Journal of Nanomaterials, vol. 2017, no. 12, p. 851034, 2017. DOI: https://doi.org/10.1155/2017/8510342
S. Majumder, P. Basnet, J. Mukherjee, and S. Chatterjee, “Effect of zinc precursor on morphology of ZnO nanoparticles,” AIP Conference Proceedings, vol. 2273, no. 1, p. 040006, 2020. DOI: https://doi.org/10.1063/5.0024934
P. K. Aspoukeh, A. A. Barzinjy, and S. M. Hamad, “Synthesis, properties and uses of ZnO nanorods: a mini review,” International Nano Letters, vol. 12, pp. 153-168, 2021. DOI: https://doi.org/10.1007/s40089-021-00349-7
A. Ejsmont, and J. Goscianska, “Hydrothermal synthesis of ZnO superstructures with controlled morphology via temperature and pH optimization,” Materials, vol. 16, p. 1641, 2023. DOI: https://doi.org/10.3390/ma16041641
H. T. T. Thuong, C. T. T. Kim, L. N. Quang, and H. Kosslick, “Highly active brookite TiO2-assisted photocatalytic degradation of dyes under the simulated solar−UVA radiation,” Progress in Natural Science: Materials International, vol. 29, pp. 641-647, 2019. DOI: https://doi.org/10.1016/j.pnsc.2019.10.001
K. Dulta, G. K. Ağçeli, P. Chauhan, R. Jasrotia, and P. K. Chauhan, “Ecofriendly synthesis of zinc oxide nanoparticles by carica papaya leaf extract and their applications,” Journal of Cluster Science, vol. 33, pp. 603-617, 2021. DOI: https://doi.org/10.1007/s10876-020-01962-w
R. Rathore, and N. Kaurav, “The structural and optical properties of ZnO nanoparticles synthesized via thermal decomposition,” Materials Today: Proceedings, vol. 54, pp. 624-627, 2022. DOI: https://doi.org/10.1016/j.matpr.2021.10.207
G. Wisz, I. Virt, P. Sagan, P. Potera, and R. Yavorskyi, “Structural, optical and electrical properties of zinc oxide layers produced by pulsed laser deposition method,” Nanoscale Research Letters, vol. 12, p. 253, 2017. DOI: https://doi.org/10.1186/s11671-017-2033-9
Y. Bai, J. Zhao, Z. Lv, and K. Lu, “Enhanced piezocatalytic performance of ZnO nanosheet microspheres by enriching the surface oxygen vacancies,” Journal of Materials Science, vol. 55, pp. 14112-14124, 2020. DOI: https://doi.org/10.1007/s10853-020-05053-z
S. D. Lokhande, M. B. Awale, and V. D. Mote, “Optical and gas sensing properties of Cu-doped ZnO nanocrystalline thin films for sensor applications,” Journal of Materials Science: Materials in Electronics, vol. 33, pp. 25063-25077, 2022. DOI: https://doi.org/10.1007/s10854-022-09213-6
T. U. D. Thi, T. T. Nguyen, Y. D. Thi, K. H. T. Thi, B. T. Phan, and K. N. Pham, “Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities,” RSC Advances, vol. 10, pp. 23899-23907, 2020. DOI: https://doi.org/10.1039/D0RA04926C
D. R. Hang, K. H. Sharma, C. H. Chen, and S. E. Islam, “Enhanced photocatalytic performance of ZnO nanorods coupled by two-dimensional α-MoO3 nanoflakes under UV and visible light irradiation” Chemistry A European Journal, vol. 22, pp. 1-9, 2016. DOI: https://doi.org/10.1002/chem.201602141
J. Jiang, Y. Li, S. Tan, and Z. Huang, “Synthesis of zinc oxide nanotetrapods by a novel fast microemulsion-based hydrothermal method,” Materials Letters, vol. 64, no. 20 pp. 2191-2193, 2010. DOI: https://doi.org/10.1016/j.matlet.2010.07.026
W. Qian, K. Zhao, D. Zhang, C. R. Bowen, Y. Wang, and Y. Yang, “Piezoelectric material-polymer composite porous foam for efficient dye degradation via the piezo-catalytic effect,” ACS Applied Materials & Interfaces, vol. 11, pp. 27862-27869, 2019. DOI: https://doi.org/10.1021/acsami.9b07857
Y. Chimupala, C. Phromma, S. Yimklan, N. Semakul, and P. Ruankham, “Dye wastewater treatment enabled by piezo-enhanced photocatalysis of single-component ZnO nanoparticles,” RSC Advances, vol. 10, no. 48, pp. 28567-28575, 2020. DOI: https://doi.org/10.1039/D0RA04746E
R. Müller, F. Huber, O. Gelme, M. Madel, J. P. Scholz, A. Minkow, U. Herr, and K. Thonke, “Chemical vapor deposition growth of zinc oxide on sapphire with methane: initial crystal formation process,” Crystal Growth & Design, vol. 19, no. 9, pp. 4964-4969, 2019. DOI: https://doi.org/10.1021/acs.cgd.9b00181
L. Nafar, R. Rasuli, M. FallahBarzoki, M. Sajadi, and M. Sajadi, “Arc‑discharge synthesis of ZnO:Ag nanoparticles for photo-catalytic applications: effects of aging, microwave radiation, and voltage,” Plasmonics, vol. 18, pp. 2305-2314, 2023. DOI: https://doi.org/10.1007/s11468-023-01916-8
S. K. Lim, S. H. Hwang, S. Kim, and H. Park, “Preparation of ZnO nanorods by microemulsion synthesis and their application as a CO gas sensor,” Sensors and Actuators B: Chemical, vol. 160, pp. 94-98, 2011. DOI: https://doi.org/10.1016/j.snb.2011.07.018
P. Rattanawarinchai, N. Khemasiri, S. Jessadaluk, C. Chananonnawathorn, M. Horprathum, A. Klamchuen, N. Kayunkid, S. Rahong, D. Phromyothin, and J. Nukeaw, “Growth time dependence on photoelectrochemical property of ZnO nanorods prepared by hydrothermal synthesis,” Surface Review and Letters, vol. 25, p. 1840001, 2018. DOI: https://doi.org/10.1142/S0218625X18400012
G. Taka, and T. D. Das, “Synthesis of ZnO nanoparticles through a simple wet chemical precipitation method,” IOP Conference Series: Earth and Environmental Science, vol. 1042, p. 012017, 2022. DOI: https://doi.org/10.1088/1755-1315/1042/1/012017
P. K. Vabbina, R. Sinha, A. Ahmadivand, M. Karabiyik, B. Gerislioglu, O. Awadallah, and N. Pala, “Sonochemical synthesis of a zinc oxide core–shell nanorod radial p–n homo-junction ultraviolet photodetector,” ACS Applied Materials & Interfaces, vol. 9, pp. 19791-19799, 2017. DOI: https://doi.org/10.1021/acsami.7b02634
J. N. Hasnidawani, H.M. Azlina, H. Norita, N. N. Bonnia, S. Ratim, and E. S. Ali, “Synthesis of ZnO nanostructures using sol-gel method,” Procedia Chemistry, vol. 19, pp. 211-216, 2016. DOI: https://doi.org/10.1016/j.proche.2016.03.095
C. Aiempanakit, T. Phantaporn, and K. Aiempanakit, “Enhanced photocatalytic activity of ZnO nanostructures deposited on mesh through electrochemical deposition and thermal oxidation,” Journal of Metals, Materials and Minerals, vol. 32, pp. 63-69, 2022. DOI: https://doi.org/10.55713/jmmm.v32i2.1254
S. Abubakar, S. T. Tan, J. Y. C. Liew, Z. A. Talib, R. Sivasubramanian, C. A. Vaithilingam, S. S. Indira, W. C. Oh, R. Siburian, S. Sagadevan, and S. Paiman, “Controlled growth of semiconducting ZnO nanorods for piezoelectric energy harvesting-based nanogenerators,” Nanomaterials, vol. 13, no. 6, pp. 1052, 2023. DOI: https://doi.org/10.3390/nano13061025
G. Yergaliuly, B. Soltabayev, S. Kalybekkyzy, Z. Bakenov, and A. Mentbayeva, “Effect of thickness and reaction media on properties of ZnO thin flms by SILAR,” Scientific Reports, vol. 12, no. 851, 2022. DOI: https://doi.org/10.1038/s41598-022-04782-2
V. V. Pokropivny, and V. V. Skorokhod, “Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science,” Materials Science and Engineering: C, vol. 27, pp. 990-993, 2007. DOI: https://doi.org/10.1016/j.msec.2006.09.023
X. Wang, M. Ahmad, and H. Sun, “Three-dimensional ZnO hierarchical nanostructures: solution phase synthesis and applications,” Materials, vol. 10, no. 11, pp. 1304, 2017. DOI: https://doi.org/10.3390/ma10111304
B. Manikandan, T. Endo, S. Kaneko, K. R. Murali, and R. John, “Properties of sol gel synthesized ZnO nanoparticles,” Journal of Materials Science: Materials in Electronics, vol. 29, pp. 9474-9485, 2018. DOI: https://doi.org/10.1007/s10854-018-8981-8
S. Al-lami, and H. Jaber, “Controlling ZnO nanostructure morphology on seedless substrate by tuning process parameters and additives,” Chemistry and Materials Research, vol. 6, no. 4, pp. 101-109, 2014.
M. A. Gatou, N. Lagopati, I. A. Vagena, M. Gazouli, and E. A. Pavlatou, “ZnO nanoparticles from different precursors and their photocatalytic potential for biomedical use,” Nanomaterials, vol. 13, pp. 122, 2023. DOI: https://doi.org/10.3390/nano13010122
J. A. A. Abdullah, A. Guerrero, and A. Romero, “Efficient and sustainable synthesis of zinc salt-dependent polycrystal zinc oxide nanoparticles: comprehensive assessment of physico-chemical and functional properties,” Applied Sciences, vol. 14, pp. 1815, 2024. DOI: https://doi.org/10.3390/app14051815
C. C. Lin, and Y. C. You, “Mass-production of ZnO nanoparticles by precipitation in a rotating packed bed: effect of zinc salt,” Journal of Materials Research and Technology, vol. 9, pp. 8451-8458, 2020. DOI: https://doi.org/10.1016/j.jmrt.2020.05.040
S. Mustapha, M. M. Ndamitso, A. S. Abdulkareem, J. O. Tijani, D. T. Shuaib, A. K. Mohammed, and A. Sumaila, “Comparative study of crystallite size using Williamson-Hall and Debye-Scherrer plots for ZnO nanoparticles,” Advances in Natural Sciences: Nanoscience and Nanotechnology, vol. 10, pp. 045013, 2019. DOI: https://doi.org/10.1088/2043-6254/ab52f7
A. K. Arora, S. Devi, V. S. Jaswal, J. Singh, M. Kinger, and V. D. Gupta, “Synthesis and characterization of ZnO nano-particles,” Oriental Journal of Chemistry, vol. 30, no.4, pp. 1671-1679, 2014. DOI: https://doi.org/10.13005/ojc/300427
V. Kumar, H. C. Swart, O. M. Ntwaeaborwa, R. E. Kroon, J. J. Terblans, S. K. K. Shaat, A. Yousif, and M. M. Duvenhage, “Origin of the red emission in zinc oxide nanophosphors,” Materials Letters, vol. 101, pp. 57-60, 2013. DOI: https://doi.org/10.1016/j.matlet.2013.03.073
A. Taufiq, H. N. Ulya, J. Utomo, Sunaryono, N. Hidayat, H. Susanto, N. Mufti, Munasir, and S. Soontaranon, “Structural, optical, and antifungal characters of zinc oxide nanoparticles prepared by sol-gel method,” Journal of Physics: Conference Series, vol. 1093, p. 012001, 2018. DOI: https://doi.org/10.1088/1742-6596/1093/1/012001
M. Aiempanakit, P. Sudjai, K. Singsumphan, S. Laksee, and C. Suwanchawalit, “Brazilein modified zinc oxide nanorods with enhanced visible light-responsive photocatalytic efficiency,” Journal of Metals, Materials and Minerals, vol. 32, pp. 70-76, 2022. DOI: https://doi.org/10.55713/jmmm.v32i2.1255
Q. Huang, and J. Liu, “Facile and clean solution synthesis of large-scale ZnO nanorods assisted with aliquat 336,” Journal of Chemistry, vol. 2013, pp. 1-6, 2013. DOI: https://doi.org/10.1155/2013/409639
Z. Jowkar, A. Moaddeli, F. Shafiei, T. Tadayon, and S. A. Hamidi, “Synthesis and characterization of mesoporous zinc oxide nanoparticles and evaluation of their biocompatibility in L929 fibroblasts,” Clinical and Experimental Dental Research, vol. 10, p. e844, 2024. DOI: https://doi.org/10.1002/cre2.844
U. Pal, C. W. Kim, N. A. Jadhav, and Y. S. Kang, “Ultrasound-assisted synthesis of mesoporous ZnO nanostructures of different porosities,” The Journal of Physical Chemistry C, vol. 113, pp. 14676-14680, 2009. DOI: https://doi.org/10.1021/jp904377n
ดาวน์โหลด
เผยแพร่แล้ว
วิธีการอ้างอิง
การอนุญาต
ลิขสิทธิ์ (c) 2024 วารสารโลหะ, วัสดุ และแร่

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












