Effects of oleylamine concentration on the synthesis of formamidinium lead halide perovskite nanocrystals and physical-optical properties
DOI:
https://doi.org/10.55713/jmmm.v34i3.2041Keywords:
Perovskite Nanocrystals, LARP, Organic Ligands, FAPbBr3Abstract
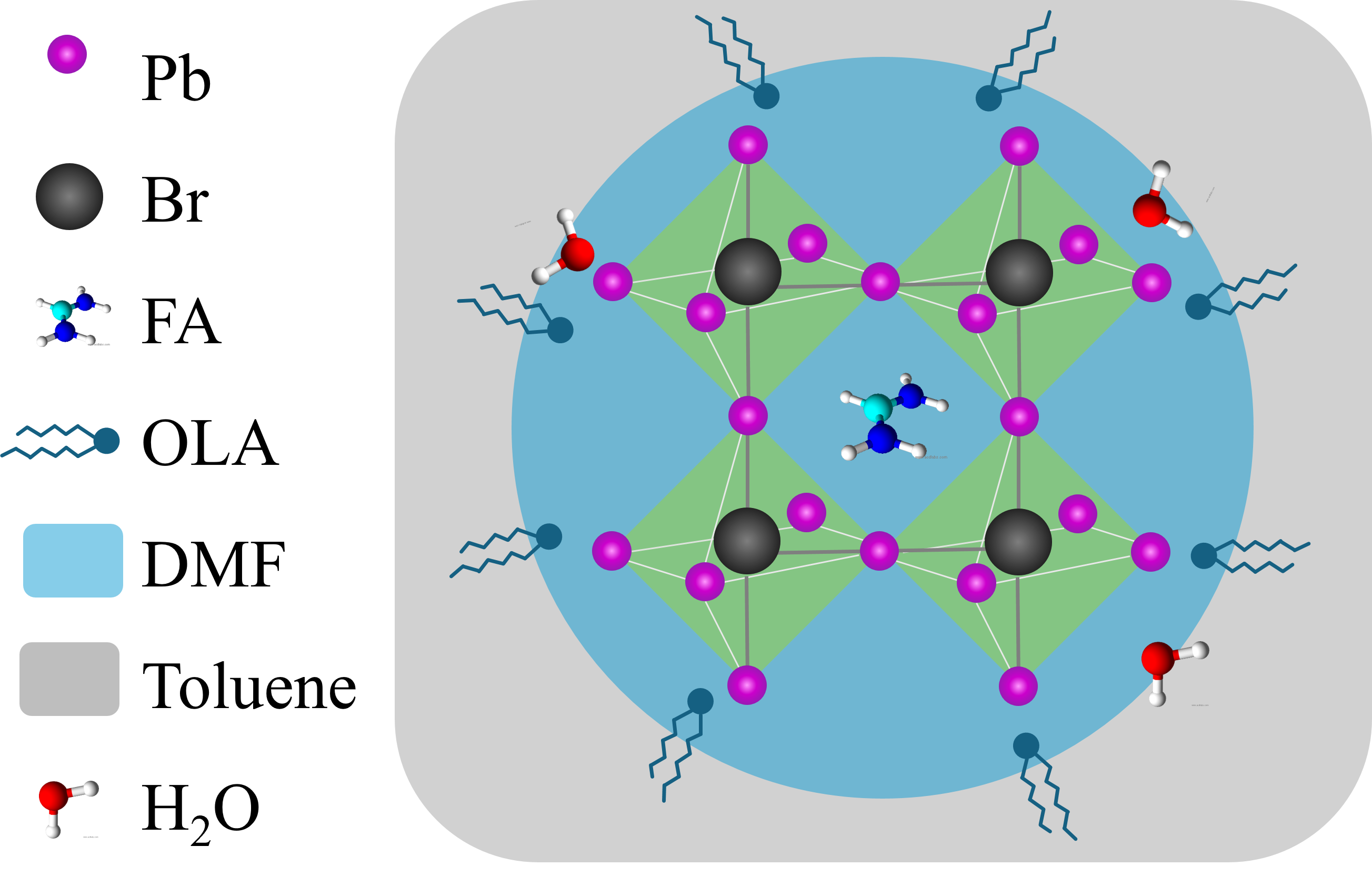
Perovskite nanocrystals (PNCs) has been extensively interested owing to their distinctive properties for applications in optoelectronics and energy harvesting. The properties of these nanocrystals, including optical and energy characteristics, can be tuned by adjusting the particle size using different synthesis techniques. Among these, the ligand-assisted reprecipitation (LARP) method has become popular for its simplicity and scalability. Nevertheless, it is vital to understand that the growth of PNCs is extremely sensitive to the conditions of synthesis, highlighting the importance of recognizing the factors that limit the formation and properties of PNCs. In this study, PNCs based on formamidinium lead bromide (FAPbBr3) were synthesized via the LARP method under room temperature and ambient atmospheric conditions. The structures and optical properties, including photoluminescence lifetime, of PNCs with varying amounts of organic ligands were investigated. Transmission electron microscopy showed that high concentrations of organic ligands lead to the formation of perovskite clusters. We also noted a slight red shift in the photoluminescence peak as the size of the PNCs increased. A peak photoluminescence quantum yield (PLQY) of 74% was achieved. This study provides crucial insights into the effects of ligand ratios and serves as a valuable resource for refining the synthesis parameters of PNCs.
Downloads
References
B. R. Sutherland, and E. H. Sargent, “Perovskite photonic sources,” Nature Photonics, vol. 10, no. 5. 2016. DOI: https://doi.org/10.1038/nphoton.2016.62
K. Lin, J. Xing, L. N. Quan, F. P. Carcia de Arquer, X, Gong, J. Lu, L, Xie, W. Zhao, D. Zhang, C. Yan, W. Li, X. Liu, Y. Lu, J. Kirman, E. H. Sargent, Q. Xiong, and Z. Wei, “Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent,” Nature, vol. 562, no. 7726, pp. 245-248, 2018. DOI: https://doi.org/10.1038/s41586-018-0575-3
F. Zhang, H. Zhong, C. Chen, X-g. Wu, X. Hu, H. Huang, J. Han, B. Zou, and Y. Dong, “Brightly luminescent and color-tunable colloidal CH3NH3PbX3 (X = Br, I, Cl) quantum dots: Potential alternatives for display technology,” ACS Nano, vol. 9, no. 4, pp. 4533-4542, 2015. DOI: https://doi.org/10.1021/acsnano.5b01154
H. Huang, L. Polavarapu, J. A. Sichert, A. S. Susha, A. S. Urban, and A. L. Rogach, “Colloidal lead halide perovskite nanocrystals: Synthesis, optical properties and applications,” NPG Asia Materials, vol. 8, no. 11, 2016. DOI: https://doi.org/10.1038/am.2016.167
M. V Kovalenko, L. Protesescu, and M. I. Bodnarchuk, “Properties and potential optoelectronic applications of lead halide perovskite nanocrystals,” Science, vol. 10, no. 358, pp. 745-750, 2017. DOI: https://doi.org/10.1126/science.aam7093
Y. S. Park, S. Guo, N. S. Makarov, and V. I. Klimov, “Room temperature single-photon emission from individual perovskite quantum dots,” ACS Nano, vol. 9, no. 10, pp. 10386-10393, 2015. DOI: https://doi.org/10.1021/acsnano.5b04584
C. T. Trinh, D. N. Minh, K. J. Ahn, Y. Kang, and K. G. Lee, “Organic-inorganic FAPbBr3 perovskite quantum dots as a quantum light source: single-photon emission and blinking behaviors,” ACS Photonics, vol. 5, no. 12, pp. 4937-4943, 2018. DOI: https://doi.org/10.1021/acsphotonics.8b01130
Y. Li, T. Ding, X. Luo, Y. Tian, X. Lu, and K. Wu, “Synthesis and spectroscopy of monodispersed, quantum-confined FAPbBr3 perovskite nanocrystals,” Chemistry of Materials, vol. 32, no. 1, pp. 549-556, 2020. DOI: https://doi.org/10.1021/acs.chemmater.9b04297
D. N. Minh, J. Kim, J. Hyon, J. H. Sim, H. H. Sowlih, C. Seo, J. Nam, S. Eom, S. Suk, S. Lee, E. Kim, and Y. Kang, “Room-temperature synthesis of widely tunable formamidinium lead halide perovskite nanocrystals,” Chemistry of Materials, vol. 29, no. 13, pp. 5713-5719, 2017. DOI: https://doi.org/10.1021/acs.chemmater.7b01705
E. Edri, S. Kirmayer, M. Kulbak, G. Hodes, and D. Cahen, “Chloride inclusion and hole transport material doping to improve methyl ammonium lead bromide perovskite-based high open-circuit voltage solar cells,” Journal of Physical Chemistry Letters, vol. 5, no. 3, pp. 429-433, 2014 DOI: https://doi.org/10.1021/jz402706q
A. Zhumekenov, M. Saodaminov, M. A. Haque, E. Alarousu, S. P. Sarmah, M. Banavoth, I. Dursun, Z-H. Miao, A. L. Abdelhady, T. Wu, O. F. Mohammed, and O. M. Bakr, “Formamidinium lead halide perovskite crystals with unprecedented long carrier dynamics and diffusion length,” ACS Energy Letters, vol. 1, no. 1, pp. 32-37, 2016. DOI: https://doi.org/10.1021/acsenergylett.6b00002
L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Bertolotti, N. Masciocchi, A. Guagliardi, and M. V. Kovalenko, “Monodisperse formamidinium lead bromide nanocrystals with bright and stable green photoluminescence,” Journal of the American Chemical Society, vol. 138, no. 43, pp. 14202-14205, 2016. DOI: https://doi.org/10.1021/jacs.6b08900
H. Bhatia, B. Ghosh, and E. Debroye, “Colloidal FAPbBr3 perovskite nanocrystals for light emission: what’s going on?,” Journal of Materials Chemistry C, vol. 10, no. 37, pp. 13437-13461, 2022. DOI: https://doi.org/10.1039/D2TC01373H
S. R. Pathipati, M. N. Shah, S. Akhil, and N. Mishra, “In situ synthesis of high-quantum-efficiency and stable bromide-based blue-emitting perovskite nanoplatelets,” Nanoscale Advances, vol. 4, no. 22, pp. 4766-4781, 2022. DOI: https://doi.org/10.1039/D2NA00354F
Y. Cai, P. Zhang, W. Bai, L. Lu, L. Wang, X. Chen, and R-J. Xie, “Synthesizing bright CsPbBr3 perovskite nanocrystals with high purification yields and their composites with in situ-polymerized styrene for light-emitting diode applications,” ACS Sustainable Chemistry Engineering, vol. 10, no. 22, pp. 7385-7393, 2022. DOI: https://doi.org/10.1021/acssuschemeng.2c01552
S. Sansoni, F. M. Anoè, and M. Meneghetti, “Simple and sustainable synthesis of perovskite-based optoelectronic material: CsPbBr3 nanocrystals via laser ablation in alcohol,” Nanoscale Advance, vol. 4, no. 23, pp. 5009-5014, 2022. DOI: https://doi.org/10.1039/D2NA00596D
V. R. Yandri, P. Wulandari, and R. Hidayat, “Photoluminescence properties of CsPbCl3 and CsPbBr3 nanocrystals synthesized by LARP method with various ligands and anti-solvents,” in Journal of Physics: Conference Series, Institute of Physics, 2022. DOI: https://doi.org/10.1088/1742-6596/2243/1/012120
L. C. Schmidt, A. Pertegas, S. Gonzalez-Carrero, O. Malinkiewicz, S. Agouram, G. M. Espallargas, H. Bolink, R. Galian, and J. Perez-Prieto, “Nontemplate synthesis of CH3NH3PbBr3 perovskite nanoparticles,” Journal og the American Chemical Society, vol. 136, no. 3, pp. 850-853, 2014. DOI: https://doi.org/10.1021/ja4109209
X. Li, Y. Wu, S. Zhang, B. Cai, Y. Gu, J. Song, and H. Zeng, “CsPbX3 quantum dots for lighting and displays: roomerature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes,” Advanced Functional Materials, vol. 26, no. 15, pp. 2435-2445, 2016. DOI: https://doi.org/10.1002/adfm.201600109
K. Abdel-Latif, F. Bateni, S. Crouse, and M. Abolhasani, “Flow synthesis of metal halide perovskite quantum dots: from rapid parameter space mapping to AI-guided modular manufacturing,” Matter, vol. 3, no. 4. Cell Press, pp. 1053-1086, 2020. DOI: https://doi.org/10.1016/j.matt.2020.07.024
L. Protesescu, S. Yakunin, O. Nazarenko, D. N. Dirin, and M. V. Kovalenko, “Low-cost synthesis of highly luminescent colloidal lead halide perovskite nanocrystals by wet ball milling,” ACS Appiedl Nano Materials, vol. 1, no. 3, pp. 1300-1308, 2018. DOI: https://doi.org/10.1021/acsanm.8b00038
L. C. Chen, Z. L. Tseng, S. Y. Chen, and S. Yang, “An ultrasonic synthesis method for high-luminance perovskite quantum dots,” Ceramics International, vol. 43, no. 17, pp. 16032-16035, 2017. DOI: https://doi.org/10.1016/j.ceramint.2017.08.066
L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh, and M V. Kovalenko, “Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut,” Nano Letters, vol. 15, no. 6, pp. 3692-3696, 2015. DOI: https://doi.org/10.1021/nl5048779
F. Krieg, S. T. Ochsenbein, S. Yakunin, S. t. Brinck, P. Aellen, A. Suess, B. Clerc, D. Guggisberg, O. Nazarenko, Y. Shynkarenko, S. Kumar, C-J. Shih, I. Infante, and M. V. Kovalenko, “Colloidal CsPbX3 (X = Cl, Br, I) nanocrystals 2.0: Zwitterionic capping ligands for improved durability and stability,” ACS Energy Letters, vol. 3, no. 3, pp. 641-646, 2018. DOI: https://doi.org/10.1021/acsenergylett.8b00035
A. Perumal, S. Shendre, M. Li, Y. K. E. Tay, V. K. Shrma, S. Chen, Z. Wei, Q. Liu, Y. Gao, P. J. S. Buenconsejo, S. T. Tan, C. L. Gan, Q. Xiong, T. C. Sum, and H. V. Demir, “High brightness formamidinium lead bromide perovskite nanocrystal light emitting devices,” Scientific Reports, vol. 6, p. 36733, 2016. DOI: https://doi.org/10.1038/srep36733
A. Prochazkova, S. Demchyshyn, C. Yumusak, J. Masilko, O. Bruggenmann, M. Weiter, M. Kaltenbrunner, N. S. Sariciftci, J. Krajcovic, Y. Salinas, A. Kovalenko, and A. Kovalenko, “Proteinogenic amino acid assisted preparation of highly luminescent hybrid perovskite nanoparticles,” ACS Applied Nano Materials, vol. 2, no. 7, pp. 4267-4274, 2019. DOI: https://doi.org/10.1021/acsanm.9b00725
H. Huang, J. Raith, S. Kershaw, S. Kalytchuk, O. Tomanec, L. Jing, A. Susha, R. Sboril, and A. Rogach, “Growth mechanism of strongly emitting CH3NH3PbBr3 perovskite nanocrystals with a tunable bandgap,” Nature Communications, vol. 8, no. 1, 2017. DOI: https://doi.org/10.1038/s41467-017-00929-2
A. Jancik Prochazkova, M. Scharber, C. Yumusak, J. Jancik, J. Masilko, O. Bruggemann, M. Weiter, N. S. Sariciftci, J. Krajcovic, Y. Salinas, and A. Kovalenko, “Synthesis conditions influencing formation of MAPbBr3 perovskite nanoparticles prepared by the ligand-assisted precipitation method,” Scientific Reports, vol. 10, no. 1, 2020. DOI: https://doi.org/10.1038/s41598-020-72826-6
F. Haydous, J. M. Gardner, and U. B. Cappel, “The impact of ligands on the synthesis and application of metal halide perovskite nanocrystals,” Journal of Materials Chemistry A, vol. 9, no. 41, Royal Society of Chemistry, pp. 23419-23443, 2021. DOI: https://doi.org/10.1039/D1TA05242J
S. Mourdikoudis, M. Menelaou, N. Fiuza, G. Zheng, S. Wei, J. Perez-Juste, P. Lakshminarayana, Z. Sofer, “Oleic acid/ oleylamine ligand pair: a versatile combination in the synthesis of colloidal nanoparticles,” Nanoscale Horizons, vol. 7, no. 9, Royal Society of Chemistry, pp. 941-1015, 2022. DOI: https://doi.org/10.1039/D2NH00111J
S. Zhou, “Rapid separation and purification of lead halide perovskite quantum dots through differential centrifugation in nonpolar solvent,” RSC Advances, vol. 11, no. 45, pp. 28410-28419, 2021. DOI: https://doi.org/10.1039/D1RA04578D
H. Zhao, H. Chen, S. Bai. C. Kuang, X. Luo, P. Teng, C. Yin, P. Zeng, L. Hou, Y. Yang, L. Duan, F. Gao, and M. Liu, “High-brightness perovskite light-emitting diodes based on FAPbBr3 nanocrystals with rationally designed aromatic ligands,” ACS Energy Letters, vol. 6, no. 7, pp. 2395–2403, 2021. DOI: https://doi.org/10.1021/acsenergylett.1c00812
I. Levchuk, A. Osvet, X. Tang, M. Brandl, J. D. Perea, F. Hoegl, G. J. Matt, R. Hock, M. Batentschuk, and C. J. Brabec, “Brightly luminescent and color-tunable formamidinium lead halide perovskite FAPbX3 (X = Cl, Br, I) colloidal nanocrystals,” Nano Letters, vol. 17, no. 5, pp. 2765-2770, 2017. DOI: https://doi.org/10.1021/acs.nanolett.6b04781
J. W. Lee, H. S. Kim, and N. G. Park, “Lewis acid-base adduct approach for high efficiency perovskite solar cells,” Accounts of Chemical Research, vol. 49, no. 2, pp. 311-319, 2016. DOI: https://doi.org/10.1021/acs.accounts.5b00440
W. S. Yang, J. Noh, N. Jeon, Y. C. Kim, S. Ryu, J. Seo, and S. Seok, “High-performance photovoltaic perovskite layers fabricated through intramolecular exchange,” Science (1979), vol. 348, no. 6240, pp. 1234-1237, 2015. DOI: https://doi.org/10.1126/science.aaa9272
J. Y. Kim, W. Jang, J. Lim, and D. H. Wang, “One-pot synthesis of all-inorganic CsPbClBr2 blue perovskite quantum dots via stoichiometric precursor,” Inorganic chemistry, vol. 62, no. 29, pp. 11665-11673, 2023. DOI: https://doi.org/10.1021/acs.inorgchem.3c01486
G. Almeida, L. Goldoni, Q. Akkerman, Z. Dang, A. H. Khan, S. Marras, I. Moreels, and L. Manna, “Role of acid-base equilibria in the size, shape, and phase control of cesium lead bromide nanocrystals,” ACS Nano, vol. 12, no. 2, pp. 1704-1711, 2018. DOI: https://doi.org/10.1021/acsnano.7b08357
K. K. Liu, Q. Liu, Y. D. Wen, Y, Liang, L. Sui, J.-Y. Sui, J.-Y. Wei, G.-W. Xue, W.-B. Zhao, X.-Y. Wu, L. Dong, and C-X. Shan, “Water-induced MAPbBr3@PbBr(OH) with enhanced luminescence and stability,” Light: Science and Applications, vol. 9, no. 1, 2020. DOI: https://doi.org/10.1038/s41377-020-0283-2
G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herz, and H. J. Snaith, “Formamidinium lead trihalide: A broadly tunable perovskite for efficient planar heterojunction solar cells,” Energy & Environmental Science, vol. 7, no. 3, pp. 982-988, 2014. DOI: https://doi.org/10.1039/c3ee43822h
M. Tabassum, Q. Zia, J. Li, M. T. Khawar, S. Aslam, and L. Su, “FAPbBr3 perovskite nanocrystals embedded in poly(l–lactic acid) nanofibrous membranes for enhanced air and water stability,” Membranes (Basel), vol. 13, no. 3, 2023. DOI: https://doi.org/10.3390/membranes13030279
Z. Zhou, S. Pang, F. Ji, B. Zhang, and G. Cui, “The fabrication of formamidinium lead iodide perovskite thin films via organic cation exchange,” Chemical Communications, vol. 52, no. 19, pp. 3828-3831, 2016. DOI: https://doi.org/10.1039/C5CC09873D
K. Hills-Kimball, Y. Nagaoka, C. Cao, E. Chaykovsky, and O. Chen, “Synthesis of formamidinium lead halide perovskite nanocrystals through solid-liquid-solid cation exchange,” Journal of Materials Chemistry C. Materials, vol. 5, no. 23, pp. 5680-5684, 2017. DOI: https://doi.org/10.1039/C7TC00598A
W. Wang, Y. Wu, D. Wang, and T. Zhang, “Effective control of the growth and photoluminescence properties of CsPbBr3/ Cs4PbBr6 nanocomposites by solvent engineering,” ACS Omega, vol. 4, no. 22, pp. 19641-19646, 2019. DOI: https://doi.org/10.1021/acsomega.9b02248
Y. L. Tong, Y. W. Zhang, K. Ma, R. Cheng, F. Wang, and S. Chen, “One-step synthesis of FA-directing FAPbBr3 perovskite nanocrystals toward high-performance display,” ACS Applied Materials & Interfaces, vol. 10, no. 37, pp. 31603-31609, 2018. DOI: https://doi.org/10.1021/acsami.8b10366
S. Zhang, and C. Wang, “Precise analysis of nanoparticle size distribution in TEM image,” Methods and Protocols, vol. 6, no. 4, p. 63, 2023. DOI: https://doi.org/10.3390/mps6040063
Y. Zu, J. Xi, L. Li, J. Dai, S. Wang, F. Yun, B. Jiao, H. Dong, X. Hou, and Z. Wu, “High-brightness and color-tunable FAPbBr3 perovskite nanocrystals 2.0 enable ultrapure green luminescence for achieving recommendation 2020 displays,” ACS Applied Materials & Interfaces, vol. 12, no. 2, pp. 2835-2841, 2020 DOI: https://doi.org/10.1021/acsami.9b18140
Downloads
Published
How to Cite
License
Copyright (c) 2024 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












