Recovery of nickel from spent electroplating solution by hydrometallurgical and electrometallurgical process
DOI:
https://doi.org/10.55713/jmmm.v32i2.1253คำสำคัญ:
recycling of nickel, spent electro nickel plating solution, hydrometallurgy, electrowinningบทคัดย่อ
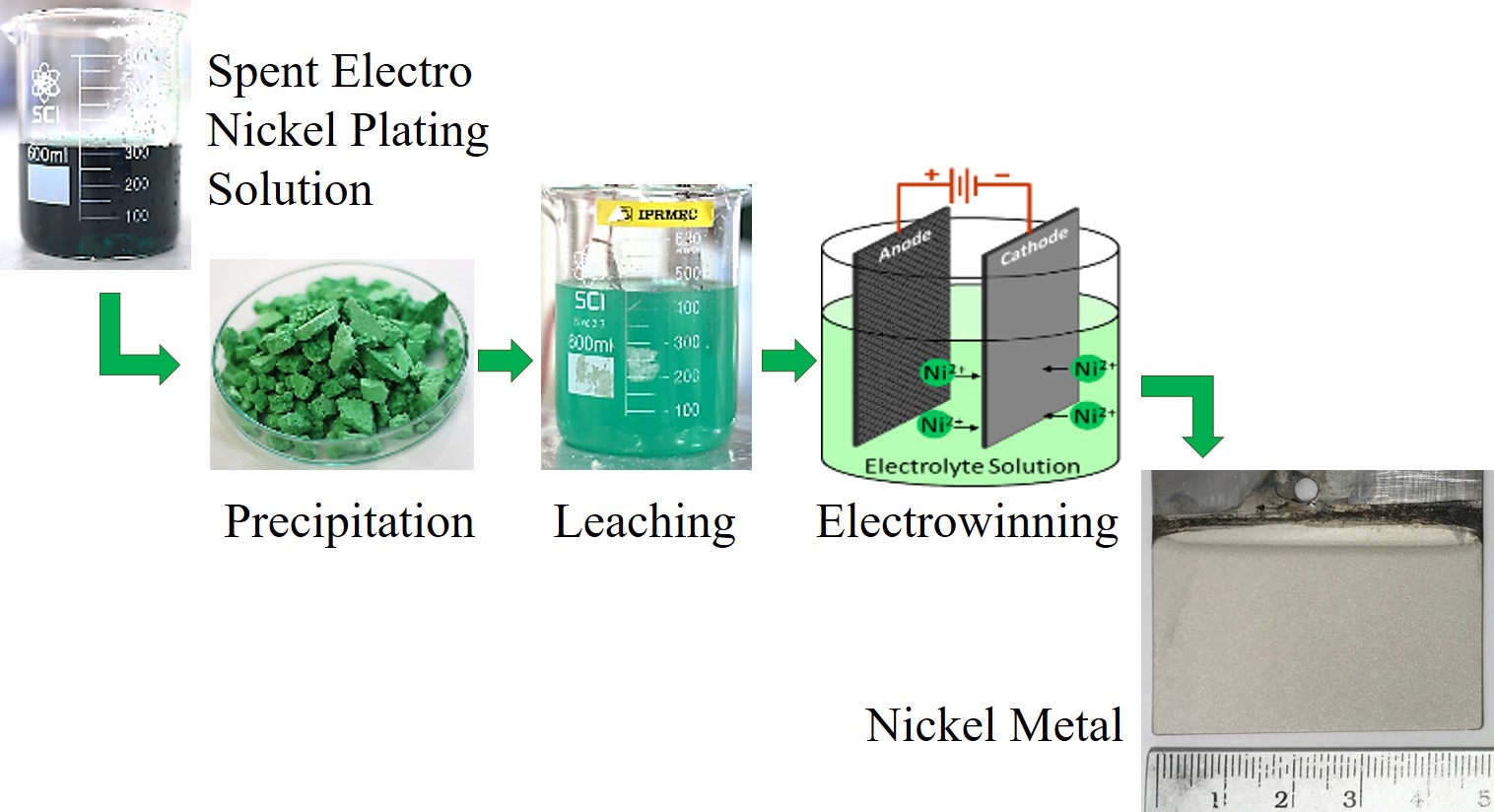
Recovery of nickel from spent electro nickel plating solution has been investigated via hydro-metallurgical and electrometallurgical processes. Experimental consisted of 3 steps, including precipitation of nickel from spent solution, leaching of nickel precipitate, and electrowinning of nickel. Nickel precipitation was performed using sodium hydroxide. Leaching parameters such as 1 M to 3 M sulfuric acid concentration, 100 g×L-1 to 300 g×L-1 solid to liquid ratio, and 30 min to 180 min leaching time were investigated. The leachate from the optimal leaching condition was further used as an electrolyte in electrowinning process. Effects of 3.3 V to 3.7 V cell voltage on recovery and purity of nickel and current efficiency of the electrowinning process were investigated. The results showed that nickel precipitation could be performed by using 2 M sodium hydroxide to adjust the solution to pH ³13. For leaching, dissolution of nickel precipitate of higher than 90% was achieved at the optimum leaching condition of 2 M sulfuric acid, solid to liquid ratio of 100:1 g×L-1 and leaching time of 60 min. Electrowinning of nickel applying the cell voltage of 3.5 V for 24 h showed the greatest nickel recovery of about 61%, giving nickel purity of 99% while the current efficiency of electrowinning cell of approximately 16% can be achieved.
Downloads
เอกสารอ้างอิง
R. Idhayachander, and K. Palanivelu, “Electrolytic Recovery of Nickel from Spent Electroless Nickel Bath Solution,” E-Journal of Chemistry, vol. 7, no. 4, pp. 1412-1420, 2010. DOI: https://doi.org/10.1155/2010/216807
D. A. Bertuol, F. D. R. Amado, H. Veit, J. Z. Ferreira, and A. M. Bernardes, “Recovery of Nickel and Cobalt from Spent NiMH Batteries by Electrowinning,” Chemical Engineering and Technology, vol. 35, no. 12, pp. 2084-2092, 2012. DOI: https://doi.org/10.1002/ceat.201200283
K. Wang, L. Li, and T. Zhang, “Synthesis of Nickel Hydroxide and Its Electrochemical Performances,” International Journal of Electrochemical Science, vol. 8, pp. 6252-6257, 2013.
J. E. Silva, D. Soares, A. P. Paiva, J. A. Labrincha, and F. Castro, “Leaching behaviour of a galvanic sludge in sulphuric acid and ammoniacal media,” Journal of Hazardous Materials, vol. 121, no. 1-3, pp. 195-202, 2005. DOI: https://doi.org/10.1016/j.jhazmat.2005.02.008
L. Li, N. Takahashi, K. Kaneko, T. Shimizu, and T. Takarada, “A novel method for nickel recovery and phosphorus removal from spent electroless nickel-plating solution,” Separation and Purification Technology, vol. 147, pp. 237-244, 2015. DOI: https://doi.org/10.1016/j.seppur.2015.04.029
N. Al-Mansi, and N. A. Monem, “Recovery of nickel oxide from spent catalyst,” Waste Management, vol. 22, no. 1, pp. 85-90, 2002.
V. Coman, B. Robotin, and P. Ilea, “Nickel recovery/removal from industrial wastes: A review,” Resources, Conservation and Recycling, vol. 73, pp. 229-238, 2013. DOI: https://doi.org/10.1016/j.resconrec.2013.01.019
C. Lupi, M. Pasquali, and A. Dell’Era, “Studies concerning nickel electrowinning from acidic and alkaline electrolytes,” Minerals Engineering, vol. 19, no. 12, pp. 1246-1250, 2006. DOI: https://doi.org/10.1016/j.mineng.2006.04.002
J. E. Silva, D. Soares, A. P. Paiva, J. A. Labrincha, and F. Castro, “Solvent extraction applied to the recovery of heavy metals from galvanic sludge,” Journal of Hazardous Materials, vol. 120, no. 1-3, pp. 113-118, 2005. DOI: https://doi.org/10.1016/j.jhazmat.2004.12.008
Y. J. Lee, S. V. Rao, B. N. Kumar, D. J. Kang, and B. R. Reddy, “Nickel recovery from spent Raneynickel catalyst through dilute sulfuric acid leaching and soda ash precipitation,” Journal of Hazardous Materials, vol. 176, no. 1-3, pp. 1122-1125, 2010. DOI: https://doi.org/10.1016/j.jhazmat.2009.11.137
F. Veglio, R. Quaresima, P. Fornari, and S. Ubaldinib, “Recovery of valuable metals from electronic and galvanic industrial wastes by leaching and electrowinning,” Waste Management, vol. 23, no. 3 pp. 245-252, 2003. DOI: https://doi.org/10.1016/S0956-053X(02)00157-5
N. M. Al-Mansi, and N. M. Abdel Monem, “Recovery of nickel oxide from spent catalyst,” Journal of Hazardous Materials, vol. 22, no. 1, pp. 85-90, 2002. DOI: https://doi.org/10.1016/S0956-053X(01)00024-1
R. Wu, M. Oliazadeh, and A. Alfantazi, “Electrical conductivity and density of NiSO4/H2SO4 solutions in the range of modern nickel electrorefining and electrowinning electrolytes,” Journal of Applied Electrochemistry, vol. 33, no. 11, pp. 1043-1047, 2003. DOI: https://doi.org/10.1023/A:1026261526134
H. Y. Lee, “Separation and Recovery of Nickel from Spent Electroless Nickel-Plating Solutions with Hydrometallurgical Processes,” Separation and Purification Technology, vol. 48, pp. 1602-1608, 2013. DOI: https://doi.org/10.1080/01496395.2012.756523
T. Benvenuti, R. S. Krapf, M. A. S. Rodrigues, A. M. Bernardes, and J. Zoppas-Ferreira, “Electrolytic Recovery of Nickel from Spent Electroless Nickel Bath Solution,” Separation and Purification Technology, vol. 129, pp. 106-112, 2014. DOI: https://doi.org/10.1016/j.seppur.2014.04.002
ดาวน์โหลด
เผยแพร่แล้ว
วิธีการอ้างอิง
ฉบับ
บท
การอนุญาต
ลิขสิทธิ์ (c) 2022 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












