Sequential injection analysis for mercury ion with modified screen – printed carbon electrode
DOI:
https://doi.org/10.55713/jmmm.v32i3.1522คำสำคัญ:
green analysis, mercury, sequential injection analysis, modified electrode, gold filmบทคัดย่อ
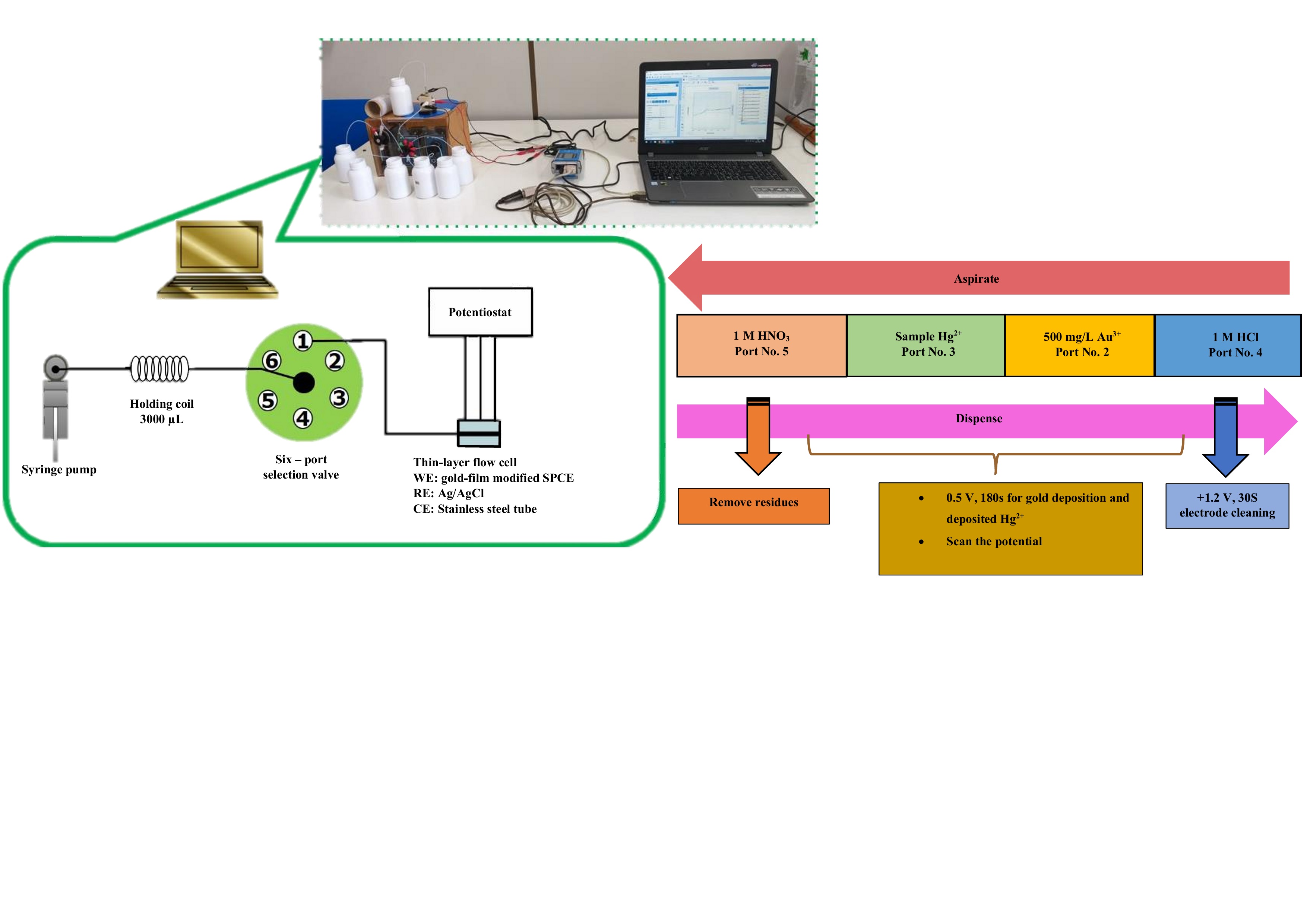
In this study, we developed a simple high-throughput and cost-effective method for monitoring toxic metal ion in an environmental aqueous sample. Mercury ion determination with Sequential Injection Analysis system (SIAs) coupled with the electrochemical detection on the modified screen-printed carbon working electrode (SPCE) is an alternative green analysis of mercury ion. The gold film was used as the modified material for improved mercury ion analysis in the automated system without memory effect on the electrode. Mercury oxidation signal was found at the potential of 0.7 V in 0.1 M HNO3 and 1.0 M HCl with the concentration low to 0.25 ± 0.18 mg×L-1. Online sample preparation and separation will study in the further experiment.
Downloads
เอกสารอ้างอิง
https://www.epa.gov/international-cooperation/mercury emissions-global-context
C. T Driscoll, R. P. Mason, H. M. Chan, D. J. Jacob, and N. Pirrone, “Mercury as a global pollutant: sources, pathways, and effects,” Environmental Science & Technology, vol. 47, p. 4967, 2013. DOI: https://doi.org/10.1021/es305071v
J. S. Marinho, M. O. Lima, E. C. de Oliveira Santos, I. M. de Jesus, M. da Conceição N. Pinheiro, C. N. Alves, and R. C. Sarkis Muller, “Mercury speciation in hair of children in three communities of the amazon, brazil,” BioMed Research International (BioMed Research International), vol. 2014, p. 1, 2014. DOI: https://doi.org/10.1155/2014/945963
R.Wang, and W. Wen-Xiong, “Importance of speciation in understanding mercury bioaccumulation in tilapia controlled by salinity and dissolved organic matter,” Environmental Science & Technology, vol. 44, p. 7964, 2010. DOI: https://doi.org/10.1021/es1011274
L. N. Suvarapu, S. Young-Kyo, and B. Sung-Ok, “Speciation and determination of mercury by various analytical techniques,” Journal Reviews in Analytical Chemistry, vol.32, p. 225, 2013. DOI: https://doi.org/10.1515/revac-2013-0003
D. O'Connor, D. Hou, Y. Sik Ok, J. Mulder, L. Duan, Q. Wu, S. Wang, F.M.G.Tack, and J. Rinkleb, “Mercury speciation transformation and transportation in soils atmospheric flux and implications for risk management: A critical review,” Environment International, vol. 126, p. 747, 2019, DOI: https://doi.org/10.1016/j.envint.2019.03.019
A. Renzoni, F. Zino, and E. Franchi, “Mercury levels along the food chain and risk for exposed populations,” Environmental Research, vol. 77, p 68, 1998. DOI: https://doi.org/10.1006/enrs.1998.3832
I. JR, H. Akagi, J. Mujumba, and C. Messo, “Environmental assessment of mercury dispersion, transformation and bioavailability in the Lake Victoria Goldfields, Tanzania.,” Journal of Environmental Management, vol. 81, p. 167, 2006. DOI: https://doi.org/10.1016/j.jenvman.2005.09.026
J. Crookand, and A. Mousavi, “The chlor-alkali process: A review of history and pollution,” Environmental Forensics, vol. 17, p. 211, 2016. DOI: https://doi.org/10.1080/15275922.2016.1177755
L. Lisa Yevugah, G. Darko, and J. Bak, “Does mercury emission from small-scale gold mining cause widespread soil pollution in Ghana,” Environmental Pollution, vol. 284, p. 116945, 2021. DOI: https://doi.org/10.1016/j.envpol.2021.116945
A. J. Clark, A. L. Labaj, J. P. Smol, L. M. Campbell, and J. Kurek, “Arsenic and mercury contamination and complex aquatic bioindicator responses to historical gold mining and modern watershed stressors in urban Nova Scotia, Canada,” Science of The Total Environment, vol.787, p. 147374, 2021 DOI: https://doi.org/10.1016/j.scitotenv.2021.147374
P. Lin, N. Fan-Hua, and L. Min-Pei, “Dietary exposure of the Taiwan population to mercury content in various seafood assessed by a total diet study,” International Journal of Environmental Research and Public Health, vol.18, p. 12227, 2021. DOI: https://doi.org/10.3390/ijerph182212227
S. Han You, S. Li Wang, W. Han Pan, W. Ching Chan, A. M Fan, and P. Lin, “Risk assessment of methylmercury based on internal exposure and fish and seafood consumption estimates in Taiwanese children,” International Journal of Hygiene and Environmental Health, vol. 221, p. 697, 2018 DOI: https://doi.org/10.1016/j.ijheh.2018.03.002
T. Dziok, M. Burya, K. Bytnar, and P. Burmistrz., “Possibility of using alternative fuels in Polish power plants in the context of mercury emissions,” Waste Management, vol. 126, p. 578, 2021. DOI: https://doi.org/10.1016/j.wasman.2021.03.053
H. Young-Seoub, K. Yu-Mi, and L. Kyung-Eun, “Methylmercury exposure and health effects,” Journal of Preventive Medicine and Public Health, vol. 45, p. 353, 2012 DOI: https://doi.org/10.3961/jpmph.2012.45.6.353
J. Hibbeln, S. Gregory, Y. Iles-Caven, C.M. Taylor, A. Emond, and J. Golding, “Total mercury exposure in early pregnancy has no adverse association with scholastic ability of the offspring particularly if the mother eats fish,” Environment International, vol. 116, p. 108, 2018. DOI: https://doi.org/10.1016/j.envint.2018.03.024
N. B. Patel, Y. Xu, L.C. McCandless, A. Chen, K. Yolton, J. Braun, R. L. Jones, Kim N. Dietrich, and B. P. Lanphear, “Very low-level prenatal mercury exposure and behaviors in children: the HOME Study,” Environmental Health, vol. 18, p. 4, 2019. DOI: https://doi.org/10.1186/s12940-018-0443-5
Health Care Without Harm. (2022, March 13). EU Mercury Regulation | Implementation tracker (2nd ed.). [Online]. Available: https://noharm-europe.org/mercury-tracker
M. Vardè, A. Servidio, G. Vespasiano, L. Pasti, A. Cavazzini, M. Di Traglia, A. Rosselli, F. Cofone, C. Apollaro, Warren R.L. Cairns, E. Scalabrin, R. De Rosa, and A. Procopio, “Ultra-trace determination of total mercury in Italian bottled waters,” Chemosphere, Impress, 2018. DOI: https://doi.org/10.1016/j.chemosphere.2018.12.020
J. Švehla, R. Žídek, T. Ružovič, K. Svoboda, and J. Kratzer, “Simple approaches to on-line and off-line speciation analysis of mercury in flue gases with detection by atomic absorption spectrometry: A pilot study,” Spectrochimica Acta Part B: Atomic Spectroscopy, vol. 156, p. 5, 2019. DOI: https://doi.org/10.1016/j.sab.2019.05.002
O. Çaylak, Ş. G. Elçi, A. Höl, A. Akdoğan, Ü. Divrikli, and L. Elçi, “Use of an aminated Amberlite XAD-4 column coupled to flow injection cold vapor generation atomic absorption spectrometry for mercury speciation in water and fish tissue samples,” Food Chemistry, vol. 274, 487, 2018. DOI: https://doi.org/10.1016/j.foodchem.2018.08.107
T. Zangmo, and A. Siripinyanond, “Exploring the applicability of nano-selenium for capture of mercury vapor: Paper based sorbent and a chemical modifier in graphite furnace atomic absorption spectrometry,” Analytica Chimica Acta, vol. 1085, p. 29, 2019. DOI: https://doi.org/10.1016/j.aca.2019.08.021
H. Zhenga, J. Hong,X. Luo, S.Li, M.Wang, B. Yanga, and M. Wang, “Combination of sequential cloud point extraction and hydride generation atomic fluorescence spectrometry for preconcentration and determination of inorganic and methyl mercury in water samples,” Microchemical Journal, vol. 145, p. 806, 2018. DOI: https://doi.org/10.1016/j.microc.2018.11.057
Jianhong Zhou, Deng Chunyan Shihui Si, Yun Shi, and Xueliang Zhao, “Study on the effect of EDTA on the photo-catalytic reduction of mercury onto nanocrystalline titania using quartz crystal microbalance and differential pulse voltammetry,” Electrochimica Acta, vol. 56, p. 2062, 2011. DOI: https://doi.org/10.1016/j.electacta.2010.11.047
S. Kulomäki, E. Lahtinen, S. Perämäki, and A. Väisänen, “Addition of thiourea and hydrochloric acid: Accurate nanogram level analysis of mercury in humic-rich natural waters by inductively coupled plasma mass spectrometry,” Talanta, vol. 218, p. 12112, 2020. DOI: https://doi.org/10.1016/j.talanta.2020.121125
K. K. Jinadasa, P. Herbello-Hermelo, E. Peña-Vázquez, P. Bermejo-Barrera, and A. Moreda-Piñeiro, “Mercury speciation in edible seaweed by liquid chromatography - Inductively coupled plasma mass spectrometry after ionic imprinted polymer-solid phase extraction,” Talanta, vol. 224, p. 121841, 2020. DOI: https://doi.org/10.1016/j.talanta.2020.121841
O. Surucu, “Electrochemical removal and simultaneous sensing of mercury with inductively coupled plasma-mass spectrometry from drinking water,” Materials Today Chemistry, vol. 23, p. 100639, 2022. DOI: https://doi.org/10.1016/j.mtchem.2021.100639
C. Jun-Liang, Y. Pei-Chia, T. Wu, and L. Yang-Wei, “Determination of mercury (II) ions based on silver-nanoparticles-assisted growth of gold nanostructures: UV–Vis and surface enhanced Raman scattering approaches,” Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, vol. 199, p. 301, 2018. DOI: https://doi.org/10.1016/j.saa.2018.03.077
C. S. Kim, N. S. Bloom, J. J. Rytuba, and G. E. Brown, “Mercury speciation by x-ray absorption fine structure spectroscopy and sequential chemical extractions: A comparison of speciation methods,” Environmental Science & Technology, vol. 37, p. 5102, 2003. DOI: https://doi.org/10.1021/es0341485
K. B. S. Perelonia, K. C. D. Benitez, R. J. S. Banicod, G. C. Tadifa, F. D. Cambia, and U. M. Montojo, “Validation of an analytical method for the determination of cadmium, lead and mercury in fish and fishery resources by graphite furnace and Cold Vapor Atomic Absorption Spectrometry,” Food Control, vol. 130, p. 108363, 2021. DOI: https://doi.org/10.1016/j.foodcont.2021.108363
D. Liu, W. Qu, W. Chen, W. Zhang, Z. Wang, and X. Jiang, “Highly sensitive, colorimetric detection of mercury(ii) in aqueous media by quaternary ammonium group-capped gold nanoparticles at room temperature,” Analytical Chemistry, vol. 82, p.198, 2010. DOI: https://doi.org/10.1021/ac1021503
C. Guan-Hua, C. Wei-Yu, Y. Yu-Chun, W. Chia-Wei, C. Huan-Tsung, and C. Chien-Fu, “Detection of mercury (II) ions using colorimetric gold nanoparticles on paper-based analytical devices,” Analytical Chemistry, vol. 86, no. 14, p. 6843, 2014. DOI: https://doi.org/10.1021/ac5008688
K. Chen, S.She, J. Zhang, A. Bayaguud, and Y. Wei, “Label-free colorimetric detection of mercury via Hg2+ ions-accelerated structural transformation of nanoscale metal-oxo clusters,” Scientific Reports, vol. 5, p. 1, 2015. DOI: https://doi.org/10.1038/srep16316
M. Lutfi, I. Fitriani, S. Wyantuti, Y.W. Hartati, R. Khaydarov, J. A. Mcalister, H. Obata, and T. Gamo, "Colorimetric detection of mercury (II) ion in aqueous solution using silver nanoparticles," Analytical Science, vol. 33, p. 831, 2017. DOI: https://doi.org/10.2116/analsci.33.831
E. Punrat, C. Maksuk, S. Chuanuwatanakul, W. Wonsawat, O. Chailapakul, “Polyaniline/graphene quantum dot-modified screen-printed carbon electrode for the rapid determination of Cr(VI) using stopped-flow analysis coupled with voltammetric technique,” Talanta, vol. 150, p.198, 2016. DOI: https://doi.org/10.1016/j.talanta.2015.12.016
L. L. Y. Qiu, Y. Feng, Y. Li, K. Wu, and L. Zhu, “Stripping voltammetric analysis of mercury ions at nitrogen-doped reduced graphene oxide modified electrode,” Journal of Electroanalytical Chemistry, vol. 865, p. 114121, 2020. DOI: https://doi.org/10.1016/j.jelechem.2020.114121
H. Reza, A. Hasanjani, and K. Zarei, "An electrochemical sensor for attomolar determination of mercury(II) using DNA/poly-L-methionine-gold nanoparticles/pencil graphite electrode," Biosensors and Bioelectronics, vol. 128, p. 1, 2019. DOI: https://doi.org/10.1016/j.bios.2018.12.039
S. Meng-Ting, Y. Xin-An, Q. Li-Ming Qin, and Z. Wang-Bing, "Highly efficient electrocatalytic vapor generation of methylmercury based on the gold particles deposited glassy carbon electrode: A typical application for sensitive mercury speciation analysis in fish samples," Analytica Chimica Acta, vol. 1025, p.58, 2018. DOI: https://doi.org/10.1016/j.aca.2018.04.057
M. Chahkandi, and M. Zargazi, “Novel method of square wave voltammetry for deposition of Bi2S3 thin film: Photocatalytic reduction of hexavalent Cr in single and binary mixtures,” Journal of Hazardous Materials, vol. 380, p. 120879, 2019. DOI: https://doi.org/10.1016/j.jhazmat.2019.120879
N. P. T.Nguyen , H. T. Nguyen , H. V. Nguyen , and L. T. Hoang, Gold–copper film electrode for voltammetry determination of mercury in water, Journal of Nanomaterials, vol. 2021, p. 1, 2021. DOI: https://doi.org/10.1155/2021/2202677
C. M. Watson, “Surface interactions of mercury on gold foil electrodes in electrodeposition and stripping and; An investigation of free thiolate ions from metal-thiolate chalcogenides," Electronic Theses and Dissertations, 2020, p.202.
B. A. Logue, and E. Manandhar, "Percent residual accuracy for quantifying goodness-of-fit of linear calibration curves," Talanta, vol. 189, p. 527, 2018. DOI: https://doi.org/10.1016/j.talanta.2018.07.046
ดาวน์โหลด
เผยแพร่แล้ว
วิธีการอ้างอิง
ฉบับ
บท
การอนุญาต
ลิขสิทธิ์ (c) 2022 วารสารโลหะ, วัสดุ และแร่

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












