Synthesis and photoluminescence properties of graphene quantum dots prepared via carbonization under pressure reduction
DOI:
https://doi.org/10.55713/jmmm.v36i1.2412คำสำคัญ:
Graphene quantum dots, Carbonization, Reduced pressure, Photoluminescence, Citric acid precursorบทคัดย่อ
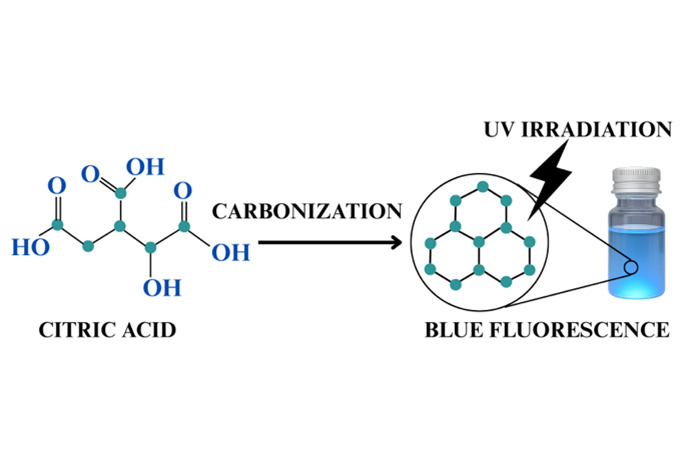
This study reports the synthesis of graphene quantum dots (GQDs) via a bottom-up carbonization approach using citric acid as a precursor under various reduced pressure conditions. The carbonization process was carried out at 250℃ in an ethanol medium, with atmospheric pressures ranging from normal to 20 inHg below atmospheric pressure. The effects of pressure reduction on the size and optical properties of GQDs were systematically investigated. Dynamic light scattering (DLS), UV–Vis spectroscopy, and fluorescence spectroscopy revealed that pressure reduction during synthesis decreased GQDs size and induced a blue-shift in both absorption and emission spectra. The optical bandgap of GQDs was tunable by varying the synthesis pressure, and strong blue fluorescence was observed under UV excitation. FT-IR analysis confirmed the formation of functional groups on the GQD surface, while the absence of epoxy-related bands indicated complete carbonization of the precursor. These findings demonstrate that pressure-controlled carbonization offers an effective method for tailoring the structural and photoluminescent properties of GQDs, enabling their application in optoelectronic and sensing devices.
Downloads
เอกสารอ้างอิง
S. Zhu, Y. Song, X. Zhao, J. Shao, J. Zhang, and B. Yang, “The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective,” Nano Research, vol. 8, no. 2, pp. 355–381, 2015. DOI: https://doi.org/10.1007/s12274-014-0644-3
H. Sun, L. Wu, W. Wei, and X. Qu, “Recent advances in graphene quantum dots for sensing,” Materials Today, vol. 16, no. 11, pp. 433–442, 2013. DOI: https://doi.org/10.1016/j.mattod.2013.10.020
S. Bak, D. Kim, and H. Lee, “Graphene quantum dots and their possible energy applications: A review,” Current Applied Physics, vol. 16, no. 9, 1192‒1201, 2016.
T. F. Yeh, C. Y. Teng, S. J. Chen, and H. Teng, “Nitrogen-doped graphene oxide quantum dots as photocatalysts for overall water-splitting under visible light illumination,” Advanced Materials, vol. 26, no. 20, pp. 3297–3303, 2014. DOI: https://doi.org/10.1002/adma.201305299
H. Liu, H. Wang, Y. Qian, J. Zhuang, L. Hu, Q. Chen, and S. Zhou, “Nitrogen-doped graphene quantum dots as metal-free photocatalysts for near-infrared enhanced reduction of 4-nitro-phenol,” ACS Applied Nano Materials, vol. 2, no. 11, pp. 7043–7050, 2019. DOI: https://doi.org/10.1021/acsanm.9b01549
S. Wongrerkdee, and P. Pimpang, “Fluorescence quenching probe based on graphene quantum dots for detection of copper ion in water,” Integrated Ferroelectrics, vol. 222, no. 1, pp. 56–68, 2022. DOI: https://doi.org/10.1080/10584587.2021.1961516
M. P. Croxall, R. T. Lawrence, R. G. Biswas, R. Soong, A. J. Simpson, and M. C. Goh, “Improved photocatalytic performance of TiO2–nitrogen-doped graphene quantum dot composites mediated by heterogeneous interactions,” The Journal of Physical Chemistry Letters, vol. 15, no. 13, pp. 3653–3657, 2024. DOI: https://doi.org/10.1021/acs.jpclett.4c00335
C. Raktham, S. Wongrerkdee, and P. Pimpang, “Utilizing nitrogen-doped graphene quantum dots to modify ZnO for enhanced photocatalytic activity in commercial insecticide degradation,” PSRU Journal of Science and Technology, vol. 9, no. 3, pp. 82–93, 2024
Z. Zhang, J. Zhang, N. Chen, and L. Qu, “Graphene quantum dots: an emerging material for energy-related applications and beyond,” Energy & Environmental Science, vol. 5, no. 10, pp. 8869–8890, 2012. DOI: https://doi.org/10.1039/c2ee22982j
D. Pan, J. Zhang, Z. Li, and M. Wu, “Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots,” Advanced Materials, vol. 22, no. 6, pp. 734–738, 2010. DOI: https://doi.org/10.1002/adma.200902825
Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou, and L. Qu, “An electrochemical avenue to green-luminescent graphene quantum dots as potential electron-acceptors for photovoltaics,” Advanced Materials, vol. 23, no. 6, pp. 776–780, 2010. DOI: https://doi.org/10.1002/adma.201003819
Y. Dong, J. Shao, C. Chen, H. Li, R. Wang, Y. Chi, X. Lin, and G. Chen, “Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid,” Carbon, vol. 50, no. 12, pp. 4738–4743, 2012. DOI: https://doi.org/10.1016/j.carbon.2012.06.002
L. Tang, R. Ji, X. Li, G. Bai, C. P. Liu, J. Hao, J. Lin, H. Jiang, K. S. Teng, Z. Yang, and S. P. Lau, “Deep ultraviolet to near-infrared emission and photoresponse in layered N-doped graphene quantum dots,” ACS Nano, vol. 8, no. 6, pp. 6312–6320, 2014.
P. Pimpang, R. Sumang, and S. Choopun, “Effect of concentration of citric acid on size and optical properties of fluorescence graphene quantum dots prepared by tuning carbonization degree,” Chiang Mai Journal of Science, vol. 45, no. 5, pp. 2005–2014, 2018.
S. Wongrerkdee, and P. Pimpang, “Ultraviolet‐shielding and water resistance properties of graphene quantum dots/polyvinyl alcohol composite-based film,” Journal of Metals, Materials and Minerals, vol. 30, no. 4, pp. 90–96, 2020. DOI: https://doi.org/10.55713/jmmm.v30i4.722
J. P. Naik, P. Sutradhar, and M. Saha, “Molecular scale rapid synthesis of graphene quantum dots (GQDs),” Journal of Nano-structure in Chemistry, vol. 7, pp. 85–89, 2017. DOI: https://doi.org/10.1007/s40097-017-0222-9
M. L. N. Oliveira, R. A. Malagoni, and M. R. Franco, “Solubility of citric acid in water, ethanol, n-propanol and in mixtures of ethanol+water,” Fluid Phase Equilibria, vol. 352, pp. 110–113, 2013. DOI: https://doi.org/10.1016/j.fluid.2013.05.014
S. Bak, D. Kim, and H. Lee, “Graphene quantum dots and their possible energy applications: A review,” Current Applied Physics, vol. 16, no. 9, pp. 1192–1201, 2016. DOI: https://doi.org/10.1016/j.cap.2016.03.026
L. Tang, R. Ji, X. Li, G. Bai, C. P. Liu, J. Hao, J. Lin, H. Jiang, K. Seng, Z. Yang, and S. P. Lau, “Deep ultraviolet to near-infrared emission and photoresponse in layered N-doped graphene quantum dots,” ACS Nano, vol. 8, no. 6, pp. 6312–6320, 2014. DOI: https://doi.org/10.1021/nn501796r
L. Song, J. Shi, J. Lu, and C. Lu, “Structure observation of graphene quantum dots by single-layered formation in layered confinement space,” Chemical Science, vol. 6, no. 8, pp. 4846–4850, 2015. DOI: https://doi.org/10.1039/C5SC01416F
S. Ahirwar, S. Mallick, and D. Bahadur, “Electrochemical method to prepare graphene quantum dots and graphene oxide quantum dots,” ACS Omega, vol. 2, no. 11, pp. 8343–8353, 2017. DOI: https://doi.org/10.1021/acsomega.7b01539
D. R. Dreyer, S. Park, C. W. Bielawski, and R. S. Ruoff, “The chemistry of graphene oxide,” Chemical Society Reviews, vol. 39, no. 1, pp. 228–240, 2010. DOI: https://doi.org/10.1039/B917103G
J.-J. Liu, Z.-T. Chen, D.-S. Tang, Y.-B. Wang, L.-T. Kang, and J.-N. Yao, “Graphene quantum dots-based fluorescent probe for turn-on sensing of ascorbic acid,” Sensors and Actuators B: Chemical, vol. 212, pp. 214–219, 2015. DOI: https://doi.org/10.1016/j.snb.2015.02.019
F. A. Permatasari, A. H. Aimon, F. Iskandar, T. Ogi, and K. Okuyama, “Role of C–N configurations in the photoluminescence of graphene quantum dots synthesized by a hydrothermal route,” Scientific Reports, vol. 6, p. 21042, 2016. DOI: https://doi.org/10.1038/srep21042
L. Wang, W. Li, B. Wu, Z. Li, S. Wang, Y. Liu, D. Pan, and M. Wu, “Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing,” Chemical Engineering Journal, vol. 300, pp. 75–82, 2016. DOI: https://doi.org/10.1016/j.cej.2016.04.123
Y. Li, H. Shu, S. Wang, and J. Wang, “Electronic and optical properties of graphene quantum dots: The role of many-body effects,” The Journal of Physical Chemistry C, vol. 119, no. 9, pp. 4983–4989, 2015. DOI: https://doi.org/10.1021/jp506969r
H. Wang, G. Liu, G. Su, H. Wei, and L. Dang, “Kinetics of ε-CL-20 during reduced pressure evaporation crystallization,” ACS Omega, vol. 10, no. 24, pp. 25581–25595, 2025. DOI: https://doi.org/10.1021/acsomega.5c01106
R. de Bruijn, J. J. Michels, and P. van der Schoot, “Transient nucleation driven by solvent evaporation,” The Journal of Chemical Physics, vol. 160, no. 8, p. 084505, 2024. DOI: https://doi.org/10.1063/5.0186395
ดาวน์โหลด
เผยแพร่แล้ว
วิธีการอ้างอิง
ฉบับ
บท
Categories
การอนุญาต
ลิขสิทธิ์ (c) 2025 วารสารโลหะ, วัสดุ และแร่

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












