A review on the development of metals-doped Vanadium oxides for zinc-ion battery
DOI:
https://doi.org/10.55713/jmmm.v34i3.2084Abstract
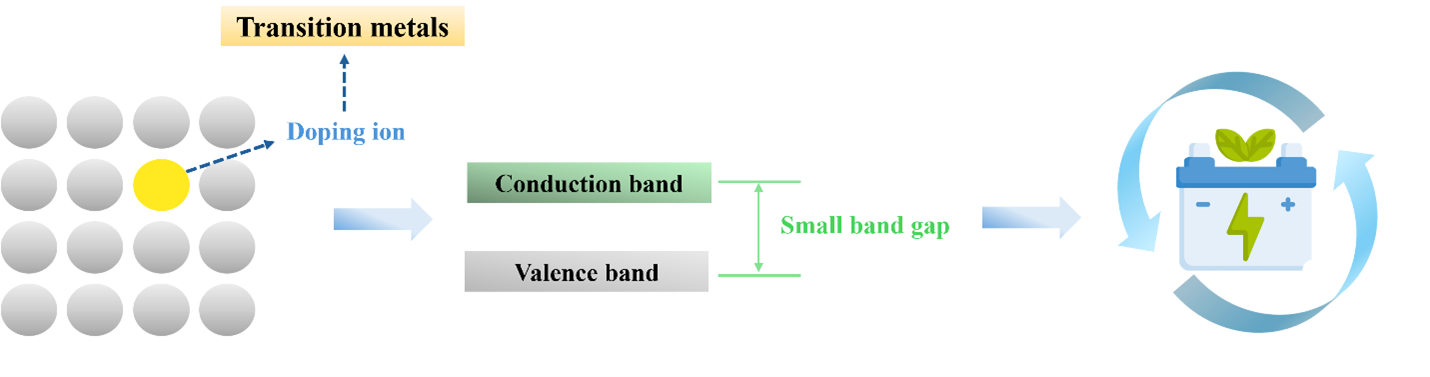
Rechargeable zinc-ion batteries (ZIBs) are emerging as promising energy storage devices for various applications, including large-scale energy storage, due to their environmental friendliness, enhanced safety, and low cost. A key challenge in ZIB development is creating cathode materials that reduce the solubility of active materials in aqueous electrolytes, increase electrical conductivity, and extend life cycles for high performance. Vanadium-based compounds, with their diverse structures and multiple oxidation states (+2, +3, +4, and +5), have been extensively studied as effective cathodes for ZIBs. This mini review highlights recent research on doping transition metals into vanadium oxide materials to achieve superior electrochemical performance compared to electrodes prepared via solid-phase synthesis and hydrothermal methods. Additionally, it offers guidance for the future development of vanadium-based materials.
Downloads
References
P. A. Owusu, and S. Asumadu-Sarkodie, "A review of renewable energy sources, sustainability issues and climate change mitigation," Cogent Engineering, vol. 3, no. 1, p. 1167990, 2016. DOI: https://doi.org/10.1080/23311916.2016.1167990
X. Jia, C. Liu, Z. Neale, J. Yang, and G. Cao, "Active materials for aqueous zinc ion batteries: Synthesis, crystal structure, morphology, and electrochemistry," Chemical Reviews, vol. 120, no. 15, pp. 7795-7866, 2020. DOI: https://doi.org/10.1021/acs.chemrev.9b00628
H. A. Kiehne, Battery technology handbook (2nd ed.). Engineering & Technology. 2003, Boca Raton: CRC Press. 542. DOI: https://doi.org/10.1201/9780203911853
B. Viswanathan, Chapter 12 - Batteries, in Energy Sources, B. Viswanathan, Editor. 2017, Elsevier: Amsterdam. p. 263-313. DOI: https://doi.org/10.1016/B978-0-444-56353-8.00012-5
J. Cao, Y. Sun, D. Zhang, D. Luo, H. Wu, X. Wang, C. Yang, L. Zhang, X. Yang, and J. Qin, "Regulating Electrode/electrolyte interface with additives towards dendrite-free zinc-ion batteries," ChemElectroChem, vol. 11, no. 13, p. e202400064, 2024. DOI: https://doi.org/10.1002/celc.202400064
J. Cao, X. Wang, D. Zhang, R. Chanojaree, L. Zhang, J. Qin, and X. Yang, "Boosting Zn metal anode stability with a dimethyl-formamide additive," Journal of Alloys and Compounds, vol. 972, p. 172773, 2024. DOI: https://doi.org/10.1016/j.jallcom.2023.172773
U. E. Solutions , The Disadvantages of Lithium-Ion Batteries for Electric Cars, in EV charging, E.y. way, Editor. 2023: https://energy5.com/the-disadvantages-of-lithium-ion-batteries-for-electric-cars.
J. Cao, J. Wu, H. Wu, Y. Jin, D. Luo, X. Yang, L. Zhang, D. Zhang, J. Qin, and J. Lu, "Dendrite-free Zinc anode via oriented plating with alkaline earth metal ion additives", Advanced Functional Materials, vol. 34, no. 32, p. 2401537, 2024. DOI: https://doi.org/10.1002/adfm.202401537
J. Cao, T. Ou, S. Geng, X. Zhang, D. Zhang, L. Zhang, D. Luo, X. Zhang, and J. Qin, "Constructing stable V2O5/V6O13 hetero-structure interface with fast Zn2+ diffusion kinetics for ultralong lifespan zinc‐ion batteries," Journal of Colloid and Interface Science, vol. 656, no. 12, p. 495-503, 2023. DOI: https://doi.org/10.1016/j.jcis.2023.11.127
F. R. McLarnon, and E. J. Cairns, "The Secondary alkaline zinc electrode," Journal of The Electrochemical Society, vol. 138, no. 2, p. 645, 1991. DOI: https://doi.org/10.1149/1.2085653
J. Cao, D. Zhang, X. Zhang, M. Sawangphruk, J. Qin, and R. Liu, "A universal and facile approach to suppress dendrite formation for a Zn and Li metal anode," Journal of Materials Chemistry A, vol. 8, no. 18, p. 9331-9344, 2020. DOI: https://doi.org/10.1039/D0TA02486D
L. F. Arenas, A. Loh, D. Trudgeon, X. Li, C. P. de Leon, and F. C. Walsh, "The characteristics and performance of hybrid redox flow batteries with zinc negative electrodes for energy storage," Renewable and Sustainable Energy Reviews, vol. 90, p. 992-1016, 2018. DOI: https://doi.org/10.1016/j.rser.2018.03.016
J. Cao, D. Zhang, R, Chanajaree, X. Zhang, X. Yang, and J. Qin, "A low-cost separator enables a highly stable zinc anode by accelerating the de-solvation effect," Chemical Engineering Journal, vol. 480, p. 147980, 2024. DOI: https://doi.org/10.1016/j.cej.2023.147980
T. Wang, C. Li, X. Xie, B. Lu, Z. He, S. Liang, and J. Zhou, "Anode Materials for aqueous zinc ion batteries: mechanisms, properties, and perspectives," ACS Nano, vol. 14, no. 12, pp. 16321-16347, 2020. DOI: https://doi.org/10.1021/acsnano.0c07041
C. Xu, B. Li, H. Du, and F. Kang, "Energetic zinc ion chemistry: The rechargeable zinc ion battery," Angewandte Chemie International Edition, vol. 51, no. 4, pp. 933-935, 2012 DOI: https://doi.org/10.1002/anie.201106307
S. Islam, M. H. Alfaruqi, V. Mathew, J. Song, S. Kim, S. Kim, J. Jo, J. P. Baboo, D. T. Pham, D. Y. Putro, Y-K. Sun, and J. Kim, "Facile synthesis and the exploration of the zinc storage mechanism of β-MnO2 nanorods with exposed (101) planes as a novel cathode material for high performance eco-friendly zinc-ion batteries," Journal of Materials Chemistry A, vol. 5, no. 44, pp. 23299-23309, 2017. DOI: https://doi.org/10.1039/C7TA07170A
M. H. Alfaruqi, V. Mathew, J. Gim, S. Kim, J. Song, J. P. Baboo, S. H. Choi, and J. Kim, "Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system," Chemistry of Materials, vol. 20, no. 10, pp. 3609-3620, 2015. DOI: https://doi.org/10.1021/cm504717p
P. Senguttuvan, S-D. Han, S. Kim, A. L. Lipson, S. Tepavcevic, T. T. Fister, I. D. Bloom, A. K. Burrell, and C. S. Johnson, "A high power rechargeable nonaqueous multivalent Zn/V2O5 battery," Advanced Energy Materials, vol. 6, no. 24, p. 1600826, 2016. DOI: https://doi.org/10.1002/aenm.201600826
P. Hu, M. Yan, T. Zhu, X. Wang, X. Wei, J. Li, Z. Liang, Z. Li, L. Chen, and L. Mai, "Zn/V2O5 aqueous hybrid-ion battery with high voltage platform and long cycle life," ACS Applied Materials and Interfaces, vol. 9, no. 49, pp. 42717-42722, 2017. DOI: https://doi.org/10.1021/acsami.7b13110
N. Zhang, Y. Dong, M. Jia, X. Bian, Y. Wang, M. Qiu, J. Xu, Y. Liu, L. Jiao, and F. Cheng, "Rechargeable aqueous Zn–V2O5 battery with high energy density and long cycle life," ACS Energy Letters, vol. 3, no. 6, pp. 1366-1372, 2018. DOI: https://doi.org/10.1021/acsenergylett.8b00565
J. Chen, G. Dawut, Y. Lu, and L Miao, "High-performance rechargeable aqueous Zn-ion batteries with a poly(benzoquinonyl sulfide) cathode," Inorganic Chemistry Frontiers, vol. 5, no. 6, pp. 1391-1396, 2018. DOI: https://doi.org/10.1039/C8QI00197A
J. Zhou, L. Shan, Z. Wu, X. Guo, F. Guozhao, and S. Liang, "Investigation of V2O5 as a low-cost rechargeable aqueous zinc ion battery cathode," Chemical Communications, vol. 54, no. 35, pp. 4457-4460, 2018. DOI: https://doi.org/10.1039/C8CC02250J
J. H. Jo, Y. K. Sun, and S. T. Myung, "Hollandite-type Al-doped VO1.52(OH)0.77 as a zinc ion insertion host material," Journal of Materials Chemistry A, vol. 5, no. 18, pp. 8367-8375, 2017. DOI: https://doi.org/10.1039/C7TA01765K
P. Y. Zavalij, F. Zhang, and M. S. Whittingham, "A new zinc pyrovanadate, Zn3(OH)2V2O7.2H2O, from X-ray powder data," Acta Crystallographica Section C: Crystal Structure Communications, vol. 53, no. 12, pp. 1738-1739, 1997. DOI: https://doi.org/10.1107/S0108270197009700
B. Sambandam, V. Soundharrajan, S. Kim, M. H. Alfaruqi, J. Jo, S. Kim, V. Mathew, Y-K. Sun, and J. Kim, "Aqueous rechargeable Zn-ion batteries: An imperishable and high-energy Zn2V2O7 nanowire cathode through intercalation regulation," Journal of Materials Chemistry A, vol. 6, no. 9, pp. 3850-3856, 2018. DOI: https://doi.org/10.1039/C7TA11237H
P. Hu, T. Zhu, X. Wang, X. Wei, M. Yan, J. Li, W. Luo, W. Yang, W. Zhang, Z. Liang, Z. Zhou, and L. Mai, "Highly durable Na2V6O16·1.63H2O nanowire cathode for aqueous zinc-ion battery," Nano Letters, vol. 18, no. 3, pp. 1758-1763, 2018. DOI: https://doi.org/10.1021/acs.nanolett.7b04889
X. Guo, F. Guozhao, W. Zhang, J. Zhou, L. Shan, L. Wang, C. Wang, T. Lin, Y. Tang, and S. Liang, "Mechanistic insights of Zn2+ storage in sodium vanadates," Advanced Energy Materials, vol. 8, no. 27, p. 1801819, 2018. DOI: https://doi.org/10.1002/aenm.201801819
B. Haüpler, C. Rossel, A. Schwenke, J. Winsberg, D. Korfer, A. Wild, and U. S. Schubert, "Aqueous zinc-organic polymer battery with a high rate performance and long lifetime," NPG Asia Materials, vol. 8, no. 7, p. e283, 2016. DOI: https://doi.org/10.1038/am.2016.82
W. Kao-ian, A. A. Mohamad, W-R. Liu, R. Pornprasetsuk, S. Siwamogsatham, and S. Kheawhom, "Stability enhancement of zinc-ion batteries using non-aqueous electrolytes," Batteries & Supercaps, vol. 5, no. 5, p. e202100361, 2022. DOI: https://doi.org/10.1002/batt.202100361
G. Li, Z. Yang, Y. Jiang, C. Jin, W. Huang, X. Ding, and Y. Huang, "Towards polyvalent ion batteries: A zinc-ion battery based on NASICON structured Na3V2(PO4)3," Nano Energy, vol. 25, pp. 211-217, 2016. DOI: https://doi.org/10.1016/j.nanoen.2016.04.051
S. D. Han, S. Kim, D. Li, V. Petkov, H. D. Yoo, P. Phillips, H. Wang, J. J. Kim, K. L. More, B. Key, R. F. Klie, J. Cabana, V. R. Stamenkovic, T. T. Fister, N. M. Markovic, A. K. Burrell, S. Tepavcevic, and J. T. Vaughey, "Mechanism of Zn insertion into nanostructured δ-MnO2: A nonaqueous rechargeable Zn metal battery," Chemistry of Materials, vol. 29, no. 11, pp. 4874-4884, 2017. DOI: https://doi.org/10.1021/acs.chemmater.7b00852
D. Kim, C. Lee, and S. Jeong, "A concentrated electrolyte for zinc hexacyanoferrate electrodes in aqueous rechargeable zinc-ion batteries" in IOP Conference Series: Materials Science and Engineering. 2018. DOI: https://doi.org/10.1088/1757-899X/284/1/012001
K. E. K. Sun, T. K. A. Hoang, T. N. L. Doan, Y. Yu, and P. Chen, "Highly sustainable zinc anodes for a rechargeable hybrid aqueous battery," Chemistry - A European Journal, vol. 24, no. 7, pp. 1667-1673, 2018. DOI: https://doi.org/10.1002/chem.201704440
K. E. K. Sun, T. K. A. Hoang, T. N. L. Doan, Y. Yu, X. Zhu, Y. Tian, and P. Chen, "Suppression of dendrite formation and corrosion on zinc anode of secondary aqueous batteries," ACS Applied Materials and Interfaces, vol. 9, no. 11, pp. 9681-9687, 2017. DOI: https://doi.org/10.1021/acsami.6b16560
J. Cao, D. Zhang, C. Gu, X. Zhang, M. Okhawilai, S. Wang, J. Han, J. Qin, and Y. Huang, "Modulating Zn deposition via ceramic-cellulose separator with interfacial polarization effect for durable zinc anode," Nano Energy, vol. 89, p. 106322, 2021. DOI: https://doi.org/10.1016/j.nanoen.2021.106322
G. Fang, J. Zhou, A. Pan, and S. Liang, "Recent advances in aqueous zinc-ion batteries," ACS Energy Letters, vol. 3, no. 10, p. 2480-2501, 2018. DOI: https://doi.org/10.1021/acsenergylett.8b01426
W. Lu, C. Xie, H. Zhang, and X. Li, "Inhibition of zinc dendrite growth in zinc-based batteries," ChemSusChem, vol. 11, no. 23, pp. 3996-4006, 2018. DOI: https://doi.org/10.1002/cssc.201801657
C. Zhai, D. Zhao, Y. He, H. Huang, B. Chen, X. Wang, and Z. Guo, "Electrolyte additive strategies for suppression of zinc dendrites in aqueous zinc-ion batteries," Batteries, vol. 8, no. 10, p. 153, 2022. DOI: https://doi.org/10.3390/batteries8100153
T. Zhou, L. Zhu, L. Xie, Q, Han, X. Yang, L. Chen, G. Wang, and X. Cao, "Cathode materials for aqueous zinc-ion batteries: A mini review," Journal of Colloid and Interface Science, vol. 605, pp. 828-850, 2022. DOI: https://doi.org/10.1016/j.jcis.2021.07.138
J. Cao, D. Zhang, R. Chanojaree, Y. Yue, Z. Zeng, X. Zhang, and J. Qin, "Stabilizing zinc anode via a chelation and desolvation electrolyte additive," Advanced Powder Materials, vol. 1, no. 1, p. 100007, 2022. DOI: https://doi.org/10.1016/j.apmate.2021.09.007
J. Cao, D. Zhang, C. Gu, X. Wang, S. Wang, X. Zhang, J. Qin, and Z-S Wu, "Manipulating crystallographic orientation of zinc deposition for dendrite-free zinc ion batteries," Advanced Energy Materials, vol. 11, no. 29, p. 2101299, 2021. DOI: https://doi.org/10.1002/aenm.202101299
S. C. Pang, S. F. Chin, and C. Y. Ling, "Controlled synthesis of manganese dioxide nanostructures via a facile hydrothermal route," Journal of Nanomaterials, vol. 2012, no. 1, p. 607870, 2012. DOI: https://doi.org/10.1155/2012/607870
J. Cao, D. Zhang, X. Zhang, Z. Zeng, J. Qin, and Y. Huang, "Strategies of regulating Zn2+ solvation structures for dendrite-free and side reaction-suppressed zinc-ion batteries," Energy & Environmental Science, vol. 15, no. 2, pp. 499-528, 2022. DOI: https://doi.org/10.1039/D1EE03377H
M. Toupin, T. Brousse, and D. Bélanger, "Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor," Chemistry of Materials, vol. 16, no. 16, pp. 3184-3190, 2004. DOI: https://doi.org/10.1021/cm049649j
Z. Wang, H. Tao, and Y. Yue, "Metal‐organic frameworks based cathodes for enhancing electrochemical performances of batteries: A review," ChemElectroChem, vol. 6, no. 21, p. 5358-5374, 2019. DOI: https://doi.org/10.1002/celc.201900843
Z. Liu, G. Pulletikurthi, and F. Endres, "A prussian blue/zinc secondary battery with a bio-ionic liquid–water mixture as electrolyte," ACS Applied Materials & Interfaces, vol. 8, no. 19, p. 12158-12164, 2016. DOI: https://doi.org/10.1021/acsami.6b01592
Y. Xue, X. Shen, H. Zhou, J. Cao, J. Pu, Z. Ji, L. Kong, and A. Yuan, "Vanadium hexacyanoferrate nanoparticles connected by cross-linked carbon nanotubes conductive networks for aqueous zinc-ion batteries," Chemical Engineering Journal, vol. 448, p. 137657, 2022. DOI: https://doi.org/10.1016/j.cej.2022.137657
F. A. Cotton, G. Wilkinson, C. A. Murillo, M. Bochmann, "Advanced inorganic chemistry" 6th edition, 1999: John Wiley & Sons. New York
V. P. Prasadam, N. Bahlawane, F. Mattelaer, G. Rampelberg, C. Detavernier, L. Fang, Y. Jiang, K. Martens, I. P. Parkin, and I. Papakonstantinou, "Atomic layer deposition of vanadium oxides: Process and application review," Materials Today Chemistry, vol. 12, p. 396-423, 2019. DOI: https://doi.org/10.1016/j.mtchem.2019.03.004
K. Kosuge, T. Takada, and S. Kachi, "Phase diagram and magnetism of V2O3—V2O5 system," Journal of the Physical Society of Japan, vol. 18, no, 2, pp. 318-319, 1963. DOI: https://doi.org/10.1143/JPSJ.18.318
A. Heidemann, K. Kosuge, Y. Ueda, and S. Kachi, "Hyperfine interaction in V3O7," Physica Status Solidi A, vol. 39, no. 1, p. K37-K40, 1977. DOI: https://doi.org/10.1002/pssa.2210390152
G. Nadkarni, and V. Shirodkar, "Experiment and theory for switching in Al/V2O5/Al devices," Thin Solid Films, vol. 105, no. 2, pp. 115-129, 1983. DOI: https://doi.org/10.1016/0040-6090(83)90200-6
G. Nagaraju, and G.T. Chandrappa, "Solution phase synthesis of Na0.28V2O5 nanobelts into nanorings and the electrochemical performance in Li battery," Materials Research Bulletin, vol. 47, no. 11, pp. 3216-3223, 2012. DOI: https://doi.org/10.1016/j.materresbull.2012.08.010
X. Zhang, D. Li, Q. Ruan, L. Liu, B. Wang, F. Xiong, C. Huang, and P. K. Chu, "Vanadium-based cathode materials for rechargeable magnesium batteries," Materials Today Energy, vol. 32, p. 101232, 2023. DOI: https://doi.org/10.1016/j.mtener.2022.101232
L. Ma, L. Na, C. Long, B. Dong, F. Daliang, Z. Liu, Y. Zhao, X. Li, J. Fan, S. Chen, S. Zhang, and C. Zhi, "Achieving both high voltage and high capacity in aqueous zinc-ion battery for record high energy density. Advanced Functional Materials, vol. 29, no. 46, p. 1906142, 2019. DOI: https://doi.org/10.1002/adfm.201906142
M. S. Javed, H. Lei, Z. Wang, B-t. Liu, X. Cai, and W. Mai, "2D V2O5 nanosheets as a binder-free high-energy cathode for ultrafast aqueous and flexible Zn-ion batteries," Nano Energy, vol. 70, p. 104573, 2020. DOI: https://doi.org/10.1016/j.nanoen.2020.104573
L. Shan, Y. Yang, W. Zhang, H. Chen, F. Guazhao, J. Zhou, and S. Liang, "Observation of combination displacement/in tercalation reaction in aqueous zinc-ion battery," Energy Storage Materials, vol. 18, p. 10-14, 2019. DOI: https://doi.org/10.1016/j.ensm.2018.08.008
U. Shankar, D. Govindarajan, C. Paul, J. Salethraj, F. J. Johanson, and M. D. Raja, "Enhanced the electrochemical properties of Ni doped V2O5 as a electrode material for supercapacitor applications," Materials Today: Proceedings, vol. 50, no. 4, pp. 2675-2678, 2022. DOI: https://doi.org/10.1016/j.matpr.2020.08.213
D. Kundu, B. D. Adams, V. Duffort, S. H. Vajargah, and L. F. Nazar, "A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode," Nature Energy, vol. 1, no. 10, p. 16119, 2016. DOI: https://doi.org/10.1038/nenergy.2016.119
Z. Wei, X. Wang, T. Zhu, P. Hu, L. Mai, and L. Zhou, "Mitigating the dissolution of V2O5 in aqueous ZnSO4 electrolyte through Ti-doping for zinc storage," Chinese Chemical Letters, vol. 35, no. 1, p. 108421, 2024. DOI: https://doi.org/10.1016/j.cclet.2023.108421
F. Ming, H. Liang, Y. Lei, S. Kandambeth, M. Eddaoudi, and H. N. Alshareef, "Layered MgxV2O5·nH2O as cathode material for high-performance aqueous zinc ion batteries," ACS Energy Letters, vol. 3, no. 10, pp. 2602-2609, 2018. DOI: https://doi.org/10.1021/acsenergylett.8b01423
Y. Yang, Y. Tang, S. Liang, Z. Wu, G. Fang, X. Cao, C. Wang, T. Lin, A. Pan, and J. Zhou, "Transition metal ion-preintercalated V2O5 as high-performance aqueous zinc-ion battery cathode with broad temperature adaptability," Nano Energy, vol. 61, pp. 617-625, 2019. DOI: https://doi.org/10.1016/j.nanoen.2019.05.005
F. Wu, Y. Wang, P. Ruan, X. Niu, D. Zheng, X. Xu, X. Gao, Y. Cai, W. Liu, W. Shi, and X. Cao, "Fe-doping enabled a stable vanadium oxide cathode with rapid Zn diffusion channel for aqueous zinc-ion batteries," Materials Today Energy, vol. 21, p. 100842, 2021. DOI: https://doi.org/10.1016/j.mtener.2021.100842
H. Cheng, X. Li, H. Hu, T. Yuan, S. Zhou, S. Dai, D. Zhang, and K. Pan, "Robust synthesis of a composite phase of copper vanadium oxide with enhanced performance for durable aqueous Zn-ion batteries," Nanotechnology Reviews, vol. 11, no. 1, pp. 1633-1642, 2022. DOI: https://doi.org/10.1515/ntrev-2022-0103
S. Zheng, J. Chen, T. Wu, R. Li, X. Zhao, Y. Pang, and Z. Pan, "Rational design of Ni-doped V2O5@3D Ni core/shell composites for high-voltage and high-rate aqueous zinc-ion batteries," Materials, vol. 17, no. 1, p. 215, 2024. DOI: https://doi.org/10.3390/ma17010215
Downloads
Published
How to Cite
License
Copyright (c) 2024 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












