Synthesis and phosphate adsorption performance of elephant dung biochar modified with magnesium and iron
DOI:
https://doi.org/10.55713/jmmm.v32i1.1243คำสำคัญ:
adsorption, biochar, elephant dung, phosphate, pyrolysisบทคัดย่อ
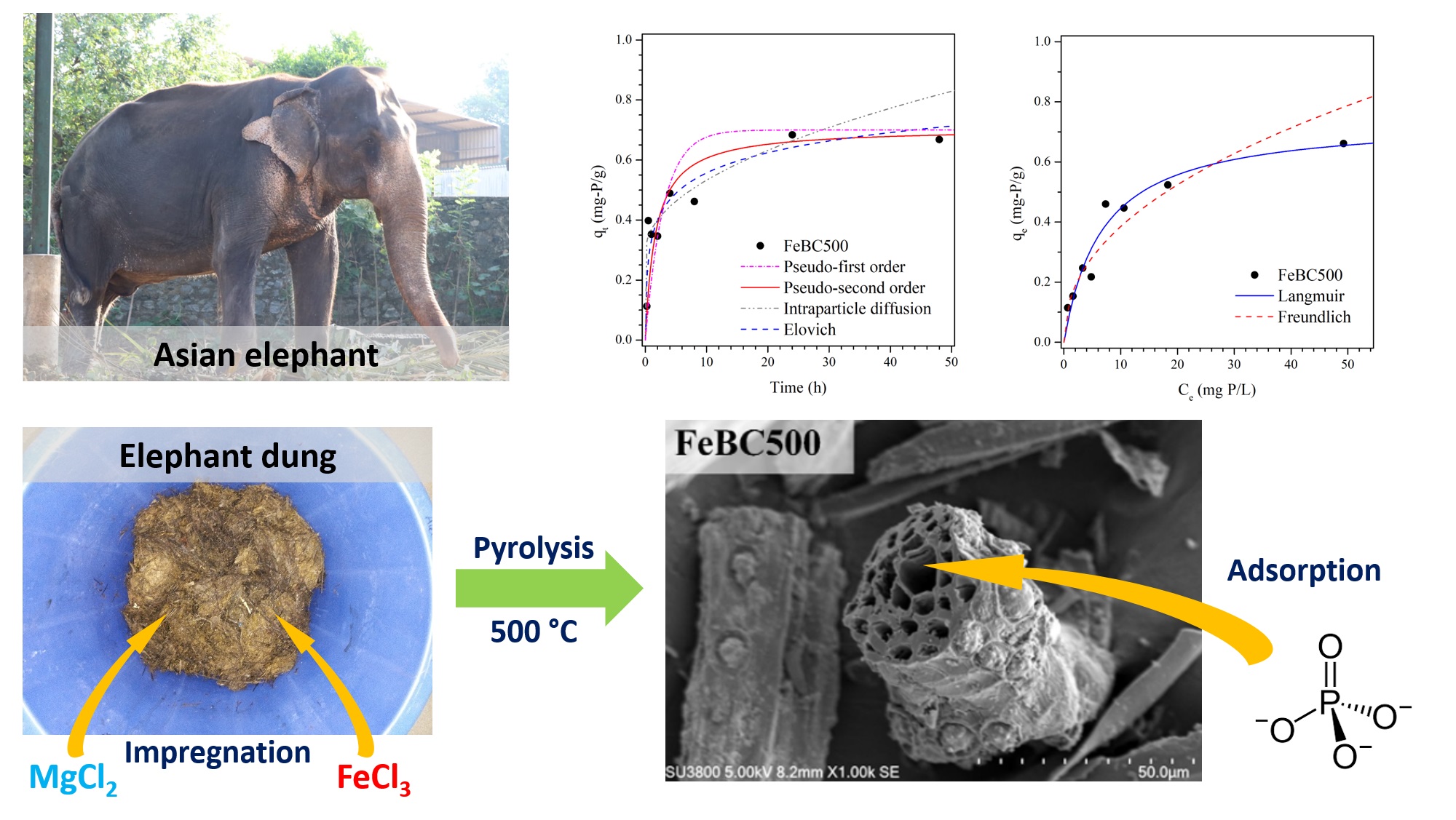
The large production volume combined with the high lignocellulose content makes elephant dung an attractive and underutilized biomass resource, but also presents waste management problems for elephant orphanages. This study explored the conversion of elephant dung into biochars by slow pyrolysis at 500°C for the recovery of phosphate. The unmodified biochar (BC500) had a specific surface area (SBET) of 62.5 m2×g-1 with point of zero charge (pHPZC) of 7.7. Biochar modification with MgCl2 (MgBC500) and FeCl3 (FeBC500) by pre-pyrolysis treatment affected the SBET (48.7 m2×g-1 and 259.4 m2×g-1, respectively) and pHPZC (8.7 and 3.3, respectively). In FeBC500, Fe was present as magnetite (Fe3O4) and hematite (α-Fe2O3) as confirmed by X-ray diffraction. Instead of adsorption, both BC500 and MgBC500 released phosphate at pH 3-6. Phosphate adsorption onto FeBC500 reached equilibrium within 24 h and followed pseudo-second order kinetics. The adsorption isotherm was best described with the Langmuir equation with a maximum adsorption capacity of 0.744 mg-P×g-1. The phosphate adsorption behavior was related to the pHPZC and metal content of the biochar. Conversion of elephant dung into biochar presents an environmentally friendly waste management solution that may find further applications in the adsorption of other nutrients or pollutants.
Downloads
เอกสารอ้างอิง
E. Chaudhary, P. Jouquet, C. Rumpel, and R. Sukumar, "Chemical parameters of decomposing dung in tropical forest as indicators of feeding behaviour of large herbivores: A step beyond classical stoichiometry," Ecological Indicators, vol. 115, pp. 106407, 2020.
P. Stępień, K. Świechowski, M. Hnat, S. Kugler, S. Stegenta-Dąbrowska, J. A. Koziel, P. Manczarski, and A. Białowiec, "Waste to carbon: Biocoal from elephant dung as new cooking fuel," Energies, vol. 12, pp. 4344, 2019.
C. Sawatdeenarunat, S. Saipa, and P. Suaisom, "Anaerobic digestion of elephant camp–derived wastes: methane potential, kinetic study, and biorefinery platform," Biomass Conversion and Biorefinery, 2021.
A. F. Saripan and A. Reungsang, "Simultaneous saccharification and fermentation of cellulose for bio-hydrogen production by anaerobic mixed cultures in elephant dung," International Journal of Hydrogen Energy, vol. 39, pp. 9028-9035, 2014.
V. Fasake and K. Dashora, "A sustainable potential source of ruminant animal waste material (dung fiber) for various industrial applications: A review," Bioresource Technology Reports, vol. 15, pp. 100693, 2021.
J. Wang and S. Wang, "Preparation, modification and environmental application of biochar: A review," Journal of Cleaner Production, vol. 227, pp. 1002-1022, 2019.
I. W. Almanassra, G. McKay, V. Kochkodan, M. Ali Atieh, and T. Al-Ansari, "A state of the art review on phosphate removal from water by biochars," Chemical Engineering Journal, vol. 409, pp. 128211, 2021.
Q. Yin, B. Zhang, R. Wang, and Z. Zhao, "Biochar as an adsorbent for inorganic nitrogen and phosphorus removal from water: A review," Environmental Science and Pollution Research, vol. 24, pp. 26297-26309, 2017.
A. El-Naggar, A. H. El-Naggar, S. M. Shaheen, B. Sarkar, S. X. Chang, D. C. W. Tsang, J. Rinklebe, and Y. S. Ok, "Biochar composition-dependent impacts on soil nutrient release, carbon mineralization, and potential environmental risk: A review," Journal of Environmental Management, vol. 241, pp. 458-467, 2019.
K. B. Cantrell, P. G. Hunt, M. Uchimiya, J. M. Novak, and K. S. Ro, "Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar," Bioresource Technology, vol. 107, pp. 419-428, 2012.
J. Zhang, B. Huang, L. Chen, Y. Li, W. Li, and Z. Luo, "Characteristics of biochar produced from yak manure at different pyrolysis temperatures and its effects on the yield and growth of highland barley," Chemical Speciation and Bioavailability, vol. 30, pp. 57-67, 2018.
Q. Chen, J. Qin, P. Sun, Z. Cheng, and G. Shen, "Cow dung-derived engineered biochar for reclaiming phosphate from aqueous solution and its validation as slow-release fertilizer in soil-crop system," Journal of Cleaner Production, vol. 172, pp. 2009-2018, 2018.
D. Wan, L. Wu, Y. Liu, H. Zhao, J. Fu, and S. Xiao, "Adsorption of low concentration perchlorate from aqueous solution onto modified cow dung biochar: Effective utilization of cow dung, an agricultural waste," Science of the Total Environment, vol. 636, pp. 1396-1407, 2018.
Y. Suma, N. Pasukphun, and N. Eaktasang, "Adsorption of methylene blue by low-cost biochar derived from elephant dung," Applied Environmental Research, vol. 43, pp. 34-44, 2021.
Y. Suma, N. Pasukphun, and N. Eaktasang, "Efficiency of biochar derived from elephant dung for adsorption of iron (III) ions," Burapha Science Journal, vol. 26, pp. 1204-1221, 2021.
C. Theivarasu and S. Chandra, "Adsorption performance of activated carbon prepared from elephant (Elephas maximus) dung for the removal of Reactive Yellow 15 from aqueous solution," Desalination and Water Treatment, vol. 51, pp. 7639-7654, 2013.
P. R. Rout, M. K. Shahid, R. R. Dash, P. Bhunia, D. Liu, S. Varjani, T. C. Zhang, and R. Y. Surampalli, "Nutrient removal from domestic wastewater: A comprehensive review on conventional and advanced technologies," Journal of Environmental Management, vol. 296, p. 113246, 2021.
Y. Zheng, A. R. Zimmerman, and B. Gao, "Comparative investigation of characteristics and phosphate removal by engineered biochars with different loadings of magnesium, aluminum, or iron," Science of the Total Environment, vol. 747, pp. 141277, 2020.
B. Singh, M. Camps-Arbestain, and J. Lehmann, Biochar: A guide to analytical methods. Boca Raton: CRC Press, 2017.
Y. Liu, X. Zhao, J. Li, D. Ma, and R. Han, "Characterization of bio-char from pyrolysis of wheat straw and its evaluation on methylene blue adsorption," Desalination and Water Treatment, vol. 46, pp. 115-123, 2012.
A. Altomare, N. Corriero, C. Cuocci, A. Falcicchio, A. Moliterni, and R. Rizzi, "QUALX2.0: A qualitative phase analysis software using the freely available database POW-COD," Journal of Applied Crystallography, vol. 48, pp. 598-603, 2015.
J. T. N. Knijnenburg, P. Kasemsiri, K. Amornrantanaworn, S. Suwanree, W. Iamamornphan, P. Chindaprasirt, and K. Jetsrisuparb, "Entrapment of nano-ZnO into alginate/polyvinyl alcohol beads with different crosslinking ions for fertilizer applications," International Journal of Biological Macromolecules, vol. 181, pp. 349-356, 2021.
K. Crombie, O. Mašek, S. P. Sohi, P. Brownsort, and A. Cross, "The effect of pyrolysis conditions on biochar stability as determined by three methods," GCB Bioenergy, vol. 5, pp. 122-131, 2013.
A. Enders and J. Lehmann, "Comparison of wet-digestion and dry-ashing methods for total elemental analysis of biochar," Communications in Soil Science and Plant Analysis, vol. 43, pp. 1042-1052, 2012.
J. T. N. Knijnenburg, K. Laohhasurayotin, P. Khemthong, and W. Kangwansupamonkon, "Structure, dissolution, and plant uptake of ferrous/zinc phosphates," Chemosphere, vol. 223, pp. 310-318, 2019.
S. Li, S. Harris, A. Anandhi, and G. Chen, "Predicting biochar properties and functions based on feedstock and pyrolysis temperature: A review and data syntheses," Journal of Cleaner Production, vol. 215, pp. 890-902, 2019.
N. Mojoudi, M. Soleimani, N. Mirghaffari, C. Belver, and J. Bedia, "Removal of phenol and phosphate from aqueous solutions using activated carbons prepared from oily sludge through physical and chemical activation," Water Science and Technology, vol. 80, pp. 575-586, 2019.
Q. Yin, M. Liu, and H. Ren, "Removal of ammonium and phosphate from water by Mg-modified biochar: Influence of Mg pretreatment and pyrolysis temperature," BioResources, vol. 14, pp. 6203-6218, 2019.
E. Wen, X. Yang, H. Chen, S. M. Shaheen, B. Sarkar, S. Xu, H. Song, Y. Liang, J. Rinklebe, D. Hou, Y. Li, F. Wu, M. Pohořelý, J. W. C. Wong, and H. Wang, "Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phytoavailability of arsenic, cadmium and lead in a paddy soil," Journal of Hazardous Materials, vol. 407, p. 124344, 2021.
J. Zhang, D. Hou, Z. Shen, F. Jin, D. O'Connor, S. Pan, Y. S. Ok, D. C. W. Tsang, N. S. Bolan, and D. S. Alessi, "Effects of excessive impregnation, magnesium content, and pyrolysis temperature on MgO-coated watermelon rind biochar and its lead removal capacity," Environmental Research, vol. 183, p. 109152, 2020.
L. Wu, S. Zhang, J. Wang, and X. Ding, "Phosphorus retention using iron (II/III) modified biochar in saline-alkaline soils: Adsorption, column and field tests," Environmental Pollution, vol. 261, p. 114223, 2020.
F. Zhang, X. Wang, J. Xionghui, and L. Ma, "Efficient arsenate removal by magnetite-modified water hyacinth biochar," Environmental Pollution, vol. 216, pp. 575-583, 2016.
J. Bedia, M. Peñas-Garzón, A. Gómez-Avilés, J. J. Rodriguez, and C. Belver, "Review on activated carbons by chemical activation with FeCl3," C—Journal of Carbon Research, vol. 6, p. 21, 2020.
Z. Xu, T. Zhang, Z. Yuan, D. Zhang, Z. Sun, Y. Huang, W. Chen, D. Tian, H. Deng, and Y. Zhou, "Fabrication of cotton textile waste-based magnetic activated carbon using FeCl3 activation by the Box–Behnken design: optimization and characteristics," RSC Advances, vol. 8, pp. 38081-38090, 2018.
S. B. Kanungo, and S. K. Mishra, "Thermal dehydration and decomposition of FeCl3·xH2O," Journal of Thermal Analysis, vol. 46, pp. 1487-1500, 1996.
H. Yang, R. Yan, H. Chen, D. H. Lee, and C. Zheng, "Characteristics of hemicellulose, cellulose and lignin pyrolysis," Fuel, vol. 86, pp. 1781-1788, 2007.
A. C. Lua, and T. Yang, "Effect of activation temperature on the textural and chemical properties of potassium hydroxide activated carbon prepared from pistachio-nut shell," Journal of Colloid and Interface Science, vol. 274, pp. 594-601, 2004.
R. R. Nair, M. M. Mondal, and D. Weichgrebe, "Biochar from co-pyrolysis of urban organic wastes—investigation of carbon sink potential using ATR-FTIR and TGA," Biomass Conversion and Biorefinery, 2020.
Z. Shen, J. Zhang, D. Hou, D. C. W. Tsang, Y. S. Ok, and D. S. Alessi, "Synthesis of MgO-coated corncob biochar and its application in lead stabilization in a soil washing residue," Environment International, vol. 122, pp. 357-362, 2019.
Z. Zhu, H. Zeng, Y. Zhu, F. Yang, H. Zhu, H. Qin, and W. Wei, "Kinetics and thermodynamic study of phosphate adsorption on the porous biomorph-genetic composite of α-Fe2O3/Fe3O4/C with eucalyptus wood microstructure," Separation and Purification Technology, vol. 117, pp. 124-130, 2013.
J. H. Park, Y. S. Ok, S. H. Kim, J. S. Cho, J. S. Heo, R. D. Delaune, and D. C. Seo, "Evaluation of phosphorus adsorption capacity of sesame straw biochar on aqueous solution: influence of activation methods and pyrolysis temperatures," Environmental Geochemistry and Health, vol. 37, pp. 969-983, 2015.
J. Qu, M. S. Akindolie, Y. Feng, Z. Jiang, G. Zhang, Q. Jiang, F. Deng, B. Cao, and Y. Zhang, "One-pot hydrothermal synthesis of NaLa(CO3)2 decorated magnetic biochar for efficient phosphate removal from water: Kinetics, isotherms, thermodynamics, mechanisms and reusability exploration," Chemical Engineering Journal, vol. 394, pp. 124915, 2020.
R. Cai, X. Wang, X. Ji, B. Peng, C. Tan, and X. Huang, "Phosphate reclaim from simulated and real eutrophic water by magnetic biochar derived from water hyacinth," Journal of Environmental Management, vol. 187, pp. 212-219, 2017.
M. Kosmulski, Surface charging and points of zero charge. Boca Raton: CRC Press, 2009.
E. J. Elzinga, and D. L. Sparks, "Phosphate adsorption onto hematite: An in situ ATR-FTIR investigation of the effects of pH and loading level on the mode of phosphate surface complexation," Journal of Colloid and Interface Science, vol. 308, pp. 53-70, 2007.
Y. Tu, Z. Peng, P. Xu, H. Lin, X. Wu, L. Yang, and J. Huang, "Characterization and application of magnetic biochars from corn stalk by pyrolysis and hydrothermal treatment," BioResources, vol. 12, pp. 1077-1089, 2016.
J. F. Lustosa Filho, C. F. Barbosa, J. S. D. S. Carneiro, and L. C. A. Melo, "Diffusion and phosphorus solubility of biochar- based fertilizer: Visualization, chemical assessment and availability to plants," Soil and Tillage Research, vol. 194, p. 104298, 2019.
M. B. Shakoor, S. Ali, M. Rizwan, F. Abbas, I. Bibi, M. Riaz, U. Khalil, N. K. Niazi, and J. Rinklebe, "A review of biochar-based sorbents for separation of heavy metals from water," International Journal of Phytoremediation, vol. 22, pp. 111-126, 2020.
ดาวน์โหลด
เผยแพร่แล้ว
วิธีการอ้างอิง
ฉบับ
บท
การอนุญาต
ลิขสิทธิ์ (c) 2022 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












