Effects of Indium and Gallium ratio on tarnish resistance, corrosion and mechanical properties of 950 silver alloy
DOI:
https://doi.org/10.55713/jmmm.v33i1.1589Keywords:
950 silver alloy, microstructure, mechanical properties, tarnish and corrosion resistance, surface analysisAbstract
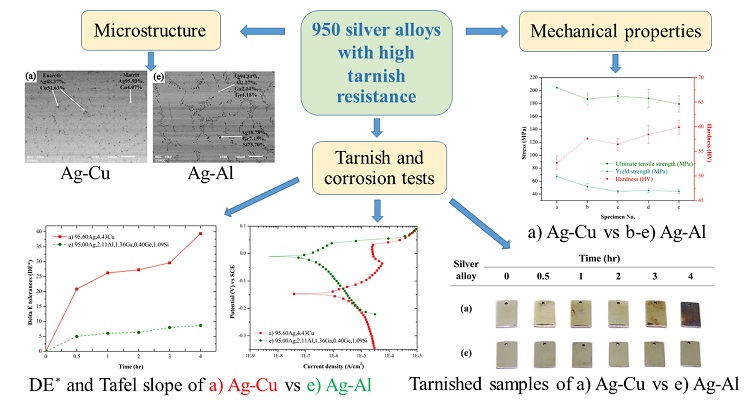
The effects of Indium (In) and Gallium (Ga) ratio on tarnish resistance, corrosion and mechanical properties of 950 silver alloy were studied. 950 Silver alloy with aluminium (Al), silica (Si) and germanium (Ge) were added with In and Ga at the range of 0.44 to 1.90 weight percent. The increment of secondary structure with Ge-Si rich phase in Ag-Al alloy increases hardness, but reduces ultimate tensile strength. The addition of In and Ga improves tarnish and corrosion resistance. The color differences as indicated by Delta E tolerances (DE*) of Ag-Al alloys are in the range of 8.64 to 11.40 while this property of Ag-Cu is 39.37. Ecorr of Ag-Al alloys are in the range of -0.068 to -0.010 V which are higher than that of Ag-Cu Alloy (-0.147 V). Besides, Ga is more effective for tarnish and corrosion resistance than In. However, Ga/In co-addition reduces these properties by the formation of Ge-Si-Ga-In phase. The protective thin film of Ag-Al alloy was detected by XPS. The Al2O3, In2O3 and Ga2O3 films were found. When the proportion of Ga in Ag-Al alloys increases, the hardness marginally increases while the tensile strength slightly reduces. The additions of Al, Ga, In, Ge and Si reduce the melting point of Ag-Al alloys comparing with Ag-Cu alloy and simultaneously improve the casting quality.
Downloads
References
C. Raub, "Use of silver in jewelry," in The Proceeding of the Santa Fe Symp of Jewelry Manufacturing Technology, 1989, pp. 241-256.
G. W. Vinal, and G. M. Schramm, "Metal industry (N.T.)," 1934, pp. 1,15,22,100,151,231.
J. P. Gamon, "Silver ternary alloy," U.S. Patent 2007009375A1, Jan. 11, 2007.
S. M. Croce, "Anti-tanish silver alloy," U.S. Patent 6841012B2, Jan. 11, 2005.
T. Cpponex, and K. P. Haung, "Silver alloy," U.S. Patent 20140271340A1, Sep. 18, 2014.
T. Cpponex and K. P. Haung, "Silver Alloy," U.S. Patent 9200350, Dec. 1, 2015.
L. H. Diamond, "Silver germanium alloy," U.S. Patent 6406664B1, Jun. 18, 2002.
M. R. Zamojski, "Silver alloy," U.S. Patent 5558833, Sep. 24, 1996.
E. Nisaratanaporn, S. Wongsriruksa, S. Pongsukitwat, and G. Lothongkum, "Study on the microstructure, mechanical properties, tarnish and corrosion resistance of sterling silver alloyed with manganese," Materials Science and Engineering: A, vol. 445-446, pp. 663-668, 2007.
C. Chanmuang, "Influence of casting techniques on hardness, tarnish behavior and microstructure of Ag-Cu-Zn-Si sterling silver jewelry alloys," Journal of Metals, Materials and Minerals, vol. 22, no.2, pp. 19-26, 2012.
S. Nisaratanaporn, and E. Nisaratanaporn, "The anti-tarnishing, microstructure analysis and mechanical properties of sterling silver with silicon addition," Journal of Metals, Materials and Minerals, vol. 12, no. 2, pp. 13-18, 2003.
E. Nisarattanaporn, "The development on silver alloy with aluminum addition for improving anti-tarnish property," The Thailand Research Fund (TRF), National Research Council of Thailand (NRCT), 2018.
Carbon X-ray photoelectron spectra [Online]. Available: https://www.thermofisher.com/th/en/home/materials-science/ learning-center/periodic-table/non-metal/carbon.html
S. Yalcin, "Enhanced copper(II) biosorption on SiO2-alginate gel composite: A mechanistic study with surface characterization," Korean Journal of Chemical Engineering, vol. 32, pp. 2116-2123, 2015.
M. Abbaszadeh, D. Krizak, and S. Kundu, Layer-by-Layer Assembly of Graphene Oxide Nanoplatelets Embedded Desalination Membranes with Improved Chlorine Resistance. 2019.
R. Romand, M. Roubin, and J. P. Deloume, "NIST x-ray photoelectron spectroscopy database," Measurement Services Division of the National Institute of Standards and Technology (NIST) Material Measurement Laboratory (MML), vol. 20899, 2000.
Y. Liu, R. G. Jordan, and S. L. Qiu, "NIST X-ray photo-electron spectroscopy database," Measurement Services Division of the National Institute of Standards and Technology (NIST) Material Measurement Laboratory (MML), vol. 20899, 2000.
F. Rueda, J. Mendialdua, A. Rodriguez, R. Casanova, Y. Barbaux, and e. al., "NIST x-ray photoelectron spectroscopy database," Measurement Services Division of the National Institute of Standards and Technology (NIST) Material Measurement Laboratory (MML), vol. 20899, 2000.
J. McKenna, J. Patel, S. Mitra, N. Soin, V. svrcek, P. Maguire, and D. Mariotti, "Synthesis and surface engineering of nano-materials by atmospheric-pressure microplasmas," The European Physical Journal Applied Physics, vol. 56, p. 24020, 2011.
R. W. Hewitt, and N. Winograd, "NIST x-ray photoelectron spectroscopy database," Measurement Services Division of the National Institute of Standards and Technology (NIST) Material Measurement Laboratory (MML), vol. 20899, 2000.
G. G. Khan, S. Ghosh, A. Sarkar, G. Mandal, G. D. Mukherjee, U. Manju, N. Banu, and B. N Dev, "Defect engineered d0 ferromagnetism in tin-doped indium oxide nanostructures and nanocrystalline thin-films," Journal of Applied Physics, vol. 118, p. 074303, 2015.
E. H. blevis, "Silicon Monoxide Properties and Evaporation Techniques," R.D. Mathis Company
M. Rateau, A. Luc, and J. P. Gamon, "Novel silver-based ternary alloy," Patent U.K. Patent GB 2255348B, 1994.
B. He, S. Liu, X. Zhao, J. Liu, Q. Ye, S. Liu, and W. Liu, "Dialkyl dithiophosphate-functionalized gallium-based liquid- metal nanodroplets as lubricant additives for antiwear and friction reduction," ACS Applied Nano Materials, vol. 3, pp. 10115-10122, 2020.
W. M. Haynes, D. R. Lide, and T. J. Bruno, CRC Handbook of Chemistry and Physics. Boca Raton: CRC press, 2014-2015.
J. A. Dean, Lange's Handbook of Chemistry. McGRAW-HILL, Inc, p. 6.112.
J. Jokkaew, "Effect of indium on mechanical properties and tarnish resistance of silicon added sterling silver," Metallurgical Engineering, Chulalongkorn University, thailand, 2001.
W. Kooratanaweich, "Effect of calcium and silicon on micro-structure, mechanical properties and tarnish resistance of Ag-Cu Alloys," Metallurgical Engineering, Chulalongkorn University, thailand, 2004.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Journal of Metals, Materials and Minerals

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Authors who publish in this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial publication in this journal.












